Abstract
A hybrid monolithic column was prepared using octavinyloctasilasesquioxane (OVS) as a monomer, benzoyl peroxide/dimethylacetamide (BPO/DMA) as initiator, ethylene glycol dimethacrylate (EDMA) as cross-linker, 1-dodecanol as porogenic agent and dimethylbenzene as cosolvent. A tidy skeleton, much bigger specific surface area (22.4 m2/g) and lower swelling property of the monolithic column with OVS added than the one without OVS added were determined with Scanning Electron Microscopy (SEM), Nitrogen adsorption/desorption measurements (BET) and swelling test with elute of different concentration of acetonitrile in water. Fourier-transform infrared spectra (FTIR) was taken to characterize the composition of groups. Moreover, a better separation performance for benzene series compounds under reversed phase liquid chromatography (RPLC) mode was obtained using the monolithic columns with OVS added than those without.
Author Contributions
Academic Editor: Dr. Praveen Kumar Sharma, Lovely Professional University, Phagwara, Punjab, India-144411
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Fengqing Wang, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Emerged in 1990s1, porous monolithic materials have been confirmed to be a good stationary phase for their straightforward fabrication, excellent permeability, high separation efficiency and other unique advantages to be used for separation of small molecules and biological matrix2, 3, 4, especially. Different from short-comings of traditional monolithic columns, the merits of hybrid monolithic columns including wide pH range tolerance, good mechanical stability, easy functionalization and high permeability enhanced its application5, 6, 7. That’s because of the inorganic and organic components in hybrid polymers being linked together by covalent bonds. Such polymers are in fact molecular composites and are produced most often by the copolymerization of a monomer with an organically functionalized inorganic compound 8. With those merits mentioned above, hybrid monoliths have been applied in both online separation fields including capillary liquid chromatography (CLC)9, capillary electro-chromatography (CEC)4, 19, etc10. However, the process to prepare hybrid monolithic, the tediously condition-sensitive sol-gel and comprehensive polymerization, encumber their development and application 9.
Polyhedral oligomeric silsesquioxanes (POSS), a series of cage-like three-dimensional oligomeric, organosilicon compounds, were used for the preparation of hybrid monoliths with free radical polymerization via “one-pot” process11, 12 instead of the tedious and uncontrollable sol-gel procedure13, 14 in recent years. The periphery consisting of cage frameworks is covalently surrounded by organic groups, such as alkyl, vinyl, ester group, phenyl etc8. Many papers about the preparation and application of hybrid monoliths with POSS added have been published. Peng and his coworkers1, for example, prepared two kinds of monolithic capillary columns with POSS added via photo- and thermally-initiated polymerization. And the POSS had been used to prepare hybrid monolith by Yao and his team members for high performance capillary electro-chromatography (CEC) 4. Zou et al., prepared several kinds of hybrid monolithic capillary columns with POSS via click reaction, thermal- and photo-initiation for the separation of small molecules 1, 9, 12, 14, 15.
In this study, a facile approach for preparation of hybrid monolithic columns with and without OVS added was adopted via thermal-initiation. Chromatographic assessments and applications were carried out to determine the chromatographic performance of the obtained monolithic columns.
Experimental Section
Materials and Instruments
Materials used in the work were AR grade except methanol and acetonitrile, which was HPLC grade. Octavinyloctasilasesquioxane (OVS) used in this study was produced from Aladdin Industrial Corporation (Shanghai, China). The reagent including acetonitrile, methanol, dimethylbenzene and 1-dodecanol were bought from Tianjin kermel chemical reagent co., Ltd. (Tianjin, China). Ethylene glycol dimethacrylate (EDMA) was purchased from Acros (New Jersey, USA). Benzoyl peroxide (BPO) and dimethylacetamide (DMA) were bought from Tianjin Guangfu Fine Chemical Research Institute
All chromatography experiments were conducted on two Thermo Unitmate 3000 system (Thermo scientific, USA) equipped with solvent delivery pumps, pump mixers, well-plate autosamplers and UV detectors. Ultrasonic cleaning machine was bought from Kun Shan Ultrasonic Instruments Co., Ltd (Jiangsu, China). Deionized water with an electrical resistivity of 18.25 mΩ/cm was prepared by a Up-pure Deionizer (Chengdu, China), and an automatic potentiometric titrator, which had been calibrated before used, was purchased from Shanghai Grows Precision Instrument Co., Ltd. (Shanghai, China). The freeze dryer was a product of Gold Sim (Gold Sim, USA). The thermostat water bath was tailor-made from Nanjing Startlab Co., Ltd. (Jiangsu, China). The vacuum drying oven with an oil pump was purchased by Shanghai Boxun Industry &Commerce Co., Ltd. (Shanghai, China)
Preparation of Poly(OVS-co-EDMA) Monolith Column
The preparation was carried out with the method of in-situ polymerization in stainless steel columns (50x4.6 mm i.d.). A certain amount of OVS was first dissolved in a certain amount of dimethylbenzene (0.30 mL), and followed with the addition of desired amount of crosslinker, porogenic solvent (EDMA and 1-dodecanol). Then with BPO (0.0045 g) added, the mixture was sonicated for 15 min under nitrogen below 30 ℃ to obtain a homogeneous solution. And finally, with addition of DMA (40 𝜇L) and vortex for 10 seconds, the polymerization mixture was poured in a stainless steel column with stoppers at the both sides to let the polymerization carry on. After being synthesized for 2.5 h at 35 ℃ in an oven, the obtained monolithic column was connected with the HPLC system and washed with ethanol (0.20 mL/min for 30 min) and methanol(10.00 mL/min for 60 min) to remove 1-dodecanol, dimethylbenzene and other soluble compounds present in the polymeric rod.
Characterization
Scanning Electron Microscopy (SEM)
The poly(OVS-co-EDMA) monolith was firstly eluted with methanol for 5h and dried under freezing for 48h. Then it was observed on a JEOL SEM 6700 microscope operating at 10kV
Fourier-Transform Infrared Spectra (FTIR)
Fourier-transform infrared spectra (FTIR) were recorded by a Varian 640-IR instrument (Varian America) with a resolution of 4 cm-1 for 32 scans over a wave number range of 4000–400 cm-1. The tablets were prepared with the mixture of KBr and samples, both of which were dried under 80 ℃ for 48h in a vacuum oven.
Nitrogen Adsorption/Desorption Measurements
The detection of specific surface area of the dry bulk monoliths was performed by nitrogen adsorption-desorption on a MicromeriticsTristarⅡ3020 (Micromeritics, USA). The samples with different amount of OVS were dried under freezing for 48h and then purged on a Micromeritics flow prep 060 (Micromeritics, USA) with nitrogen for 6 h under 50 ℃..
Result and Discussion
Optimization for the Preparation Conditions of Monolithic Columns
Single factor experiment was introduced for the optimization conditions to prepare poly(OVS-co-EDMA) hybrid monolithic column. With the consideration that the ratio between crosslinker, monomer and porogenic agent representing great affection to the polymer skeleton and also its chromatographic behaviors, several columns were prepared under different ratios between EDMA, OVS and 1-dodecanol illustrated in Table 1. Back pressure was adopted to compare the chromatographic performance of the monolithic column. Firstly, experiments from column A to column E were used to optimize the ratios between crosslinker and porogenic agent (EDMA and 1-dodecanol) ranging from 1:3 to 1:1 with constant bulk volume (1.60 mL). It could be seen that the more 1-dodecanol added, the softer material was obtained. Even though the mixture of column B could polymerize, the OVS precipitated from the mixture leading to an uneven monolith which is useless. This can be explained by much lower solubility of OVS in 1-dodecanol. Under the proper amount of crosslinker and porogenic agent, different amount of OVS (ranging from 0 mg to 40 mg) was added to investigate the effect of OVS to the columns formation. While, comparing the results from column R to column N, the mechanical strength of composite was getting stronger and the back pressure decreased at the beginning and then increased when more and more OVS was added. The phenomenon may be due to the inorganic material enhancing the mechanical properties of hybrid monoliths. The reduction of pressure along column with 20 mg OVS (column P) may because of the disordered agglomerated organic globules (as seen for column R) turned into innerframe structure. In Figure 1, the column P possessed not only higher permeability (1.2305x10-12cm2 for column P, 0.9829 x10-12cm2 for column R) but also higher pressure endurance than that of column R. While, except the role in skeleton structure, another function of OVS during polymerization was nucleation. When the amount of OVS added being more than 30mg, the nucleation of OVS created smaller size of skeleton and more pyknotic accumulation of monolithic polymer in microscopic size.
Table 1. Conditions optimization for the preparation of poly(OVS-co-EDMA) hybrid monolithic columns| Column | EDMA (ml) | 1-Dodecanol (ml) | Dimethylbenzene (ml) | OVPOSS (mg) | BPO/DMA (mg/µl) | Mechanical/ Physical properties (sa,nb,hc,dd) | Backpressuree(bar) |
| A | 0.40 | 1.20 | 0.30 | 20 | 4.5/40 | d | x |
| B | 0.50 | 1.10 | 0.30 | 20 | s | x | |
| C | 0.60 | 1.00 | 0.30 | 20 | n | 7 | |
| D | 0.70 | 0.90 | 0.30 | 20 | hh | 14 | |
| E | 0.80 | 0.80 | 0.30 | 20 | hhh | 19 | |
| F | 0.55 | 1.05 | 0.30 | 30 | d | x | |
| G | 0.55 | 1.05 | 0.30 | 20 | n | 8 | |
| H | 0.55 | 1.05 | 0.30 | 10 | n | 7 | |
| I | 0.55 | 1.05 | 0.30 | 0 | s | x | |
| J | 0.6 | 1.00 | 0.30 | 40 | d | x | |
| K | 0.6 | 1.00 | 0.30 | 30 | h | 9 | |
| L | 0.6 | 1.00 | 0.30 | 10 | n | 7 | |
| M | 0.6 | 1.00 | 0.30 | 0 | s | x | |
| N | 0.65 | 0.95 | 0.30 | 40 | hhh | 20 | |
| O | 0.65 | 0.95 | 0.30 | 30 | hh | 15 | |
| P | 0.65 | 0.95 | 0.30 | 20 | h | 11 | |
| Q | 0.65 | 0.95 | 0.30 | 10 | n | 8 | |
| R | 0.65 | 0.95 | 0.30 | 0 | n | 12 |
Figure 1.Plot of the pressure drop along the home-made monolithic column. The mobile phase was a mixture of acetonitrile and water (65/35, v/v) and temperature was 25±0.1℃ at flow rate between 0.10 and 2.50mL/min.The backpressure removed the viscosity influence of elutes.
Opinions above could be illustrated in Figure 2. The effect of increasing the amount of OVS (column R, P, N) was observed using SEM and BET. More morphology was obtained for monolith with OVS (20 mg) than without. Even though larger specific surface area could be observed in column N, the tidy skeleton in proper size of column P was much more suitable for preparation of efficient monolithic column. The auxiliary skeleton observed for column N decreases the uniformity and controllability during the polymerization leading to lower chromatographic performance. The condition of column P will be used for further experiment.
Figure 2.Polymer skeleton and specific surface area of monolithic columns with different amount of OVS tested with SEM and BET.
FTIR was used to confirm the group composition in the monolith with and without OVS added. The result was shown in Figure 3. The peaks associated with groups are C-H (2990-2958 cm-1), C=O (1690-1729 cm-1), Si-O-Si (1102 cm-1) and –OH (3500 cm-1)18.
Figure 3.Difference between monolith with OVS (redline) and without OVS (blue-line) added based on FT-IR spectra.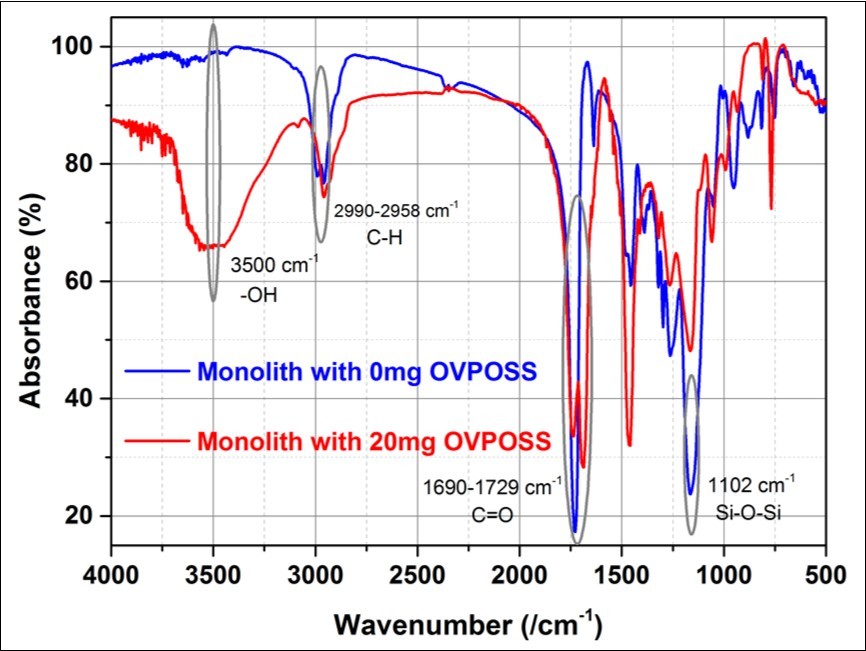
Swelling Properties
In liquid chromatography, the mobile phase can diffuse into and even swell the polymer monolith. It means that the volume and morphology of monolithic column can be different between the monolith in dry and wet state16.
The swelling properties of the obtained monolithic column with and without OVS added was measured using different acetonitrile-water mixtures as the mobile phase. Thiourea, a non-retained compound for reversed-phase liquid chromatography, was injected to measure the porosity of monolith18. The elution volumes of thiourea under different concentration of acetonitrile in water for column P and R were illustrated in Figure 4Ⅰcurve A and curve B, respectively. The result demonstrated that the elution volumes of thiourea were almost the same (0.5789 mL for column R and 0.5741 mL for column P) when the concentration of acetonitrile was above 25%, during which a classical reverse phase liquid chromatography (RPLC) has been proved because of the non-retention property of thiourea along the monolith. The porosity of both monolith (69.7% for column R and 69.1% for column P) could be calculated from the elution volume of thiourea. While, a bigger swelling property of column R than column P could be seen from the sharp raise and drop appearance in curve C, which was calculated by subtracting curve B from curve A. The trend of curve C in figure 4Ⅱwas also calculated from curve A and curve B, whichwere the plots of back pressure with the concentration of acetonitrile in water raising from 0% to 100%. The tendency of both column P and column R were almost the same. The highest back pressure detected for both column R and column P were taken place with 80% acetonitrile in water as the elution. The elution volume of thiourea using 80% acetonitrile was smaller (about 4.1% and 1.5%) than 100% acetonitrile for both column R and column P (the dead volume of the chromatography system was removed). The result means that both columns swelled and the swelling of column P was smaller than the column R. The phenomenon above could be explained by the stronger rigidity of monolith skeleton with OVS than without OVS limited the swelling of polymers
Figure 4.Top graph: changes in elution volume with changing composition of aqueous acetonitrile mobile phase. Curve A: the elution volume of thiourea for column P, curve B: the elution volume of thiourea for column R and curve C: calculated by subtracting curve B from curve A. Bottom graph: changes in backpressure with changing composition of aqueous acetonitrile mobile phase. Curve A: the pressure drop for column P, curve B: the pressure drop for column R and curve C: calculated by subtracting curve B from curve A. Experiment conditions were: elute was mixed from different concentration of acetonitrile and water, the temperature was 25±0.1℃ and flow rate was 1.00mL/min at the wavelength of 262 nm.
Plate Height along the Flow Rate
Plate height of the obtained monolithic column along flow rate was examined with the elute of acetonitrile and water (75/25, v/v) at 25℃. Five compounds (1,2-Diaminobenzene, p-Nitrophenol, (Dichloromethyl)benzene, Naphthalenol, 1-Aminonaphthalene) were used to examine the performance of obtained monolithic column, which is shown in Figure 5. Comparing the plate height under different flow rates, the smallest plate height of most compounds were taken place around flow rate from 0.80 to 1.20 mL/min except for 1-Aminonaphthalene, the best chromatographic performance was at the flow rate of 0.60 mL/min. While, taken the separation time into consideration, a shorter time was cost for the flow rate of 1.00 mL/min than the one of 0.80mL/min, it could be seen fromFigure 6. But the chromatographic performance was almost the same (16800plate/m for 1,2-Diaminobenzene at 0.80mL/min and 16100plate/m at 1.00mL/min). The baseline separation for p-Nitrophenol and (Dichloromethyl) benzene was not achieved, when the flow rate was increased to 1.20mL/min.
Fully consideration above, the flow rate of 1mL/min was taken for the following experiments.
Figure 5.Plate height of different compounds recorded from column P under different flow rate. Experiment conditions were: the elute was a mixture of acetonitrile and water (75/25, v/v) and temperature was 25℃.
Figure 6.Chromatographic performance of column P under different flow rates (0.80, 1.00 and 1.20mL/min). The compounds are: 1: 1,2-Diaminobenzene, 2: p-Nitrophenol, 3: (Dichloromethyl)benzene, 4: Naphthalenol, 5: 1-Aminonaphthalene.
Application of Poly(OVS-co-EDMA) Monolithic Columns
To compare the chromatographic performance of the home-made monolithic column with OVS with without OVS added, several small molecules including neutral, basic and acidic compounds were used as the test compounds. The eluting sequence of molecules on both columns were: 1,2-Diaminobenzene > p-Nitroaniline > Benzene > 1-Naphthylamine > 4-Chloronitrobenzene > Naphthalene > Diphenyl > Phenanthrene. From Figure 7, it could be seen that a much better separation was obtained for the column with OVS added than the one without OVS contended.
Figure 7.Small molecules separated via monolithic column with and without OVS added. Compounds were: 1:1,2-Diaminobenzene, 2:p-Nitroaniline, 3:Benzene, 4:1-Naphthylamine, 5:4-Chloronitrobenzene, 6:Naphthalene, 7:Diphenyl, 8:Phenanthrene. Experiment conditions were: the elute was a mixture of acetonitrile and water (65/35, v/v), temperature was 25℃ and flow rate was 1.00mL/min.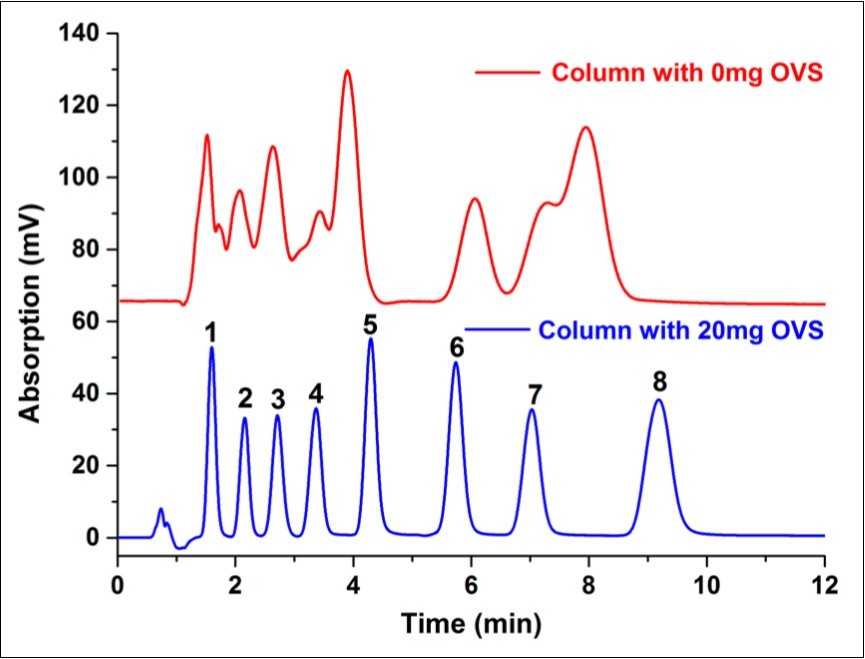
Conclusion
A new kind of hybrid organic-silica monolithic column was prepared successfully with OVS added as a monomer. Three different polymer skeletons were obtained with different amount of monomer added. The monolith with OVS added showed stronger rigidity. The classical RPLC mode was observed during the experiment of swelling test. Under the optimization conditions, small molecules were separated successfully.
Acknowledge
The authors would like to gratefully acknowledge financial support received from the National Natural Science Foundation of China (no. 21175031, 21575033, 21505030), the Natural Science Foundation of Hebei Province (no. B2013201082, B2015201024) and the Natural Science Foundation of Hebei University (no. 2014-05).
References
- 1.Wang Hongwei, Ou Junjie, Liu Zhongshan, Lin Hui, Peng Xiaojun et al. (2015) Chromatographic Efficiency Comparison of Polyhedral Oligomeric Silsesquioxanes-Containing Hybrid Monoliths Via Photo-and Thermally-Initiated Free-Radical Polymerization in Capillary Liquid Chromatography for Small Molecules’,Journal of Chromatography A,1410. 110-17.
- 2.Chen Yingzhuang, Wu Minghuo, Wang Keyi, Chen Bo, Yao Shouzhuo et al. (2011) Vinyl Functionalized Silica Hybrid Monolith-Based Trypsin Microreactor for on Line Digestion and Separation Via Thiol-Ene “Click” Strategy’,Journal of Chromatography A,1218. 7982-88.
- 3.Lin Hui, Ou Junjie, Zhang Zhenbin, Dong Jing, Wu Minghuo et al. (2012) Facile Preparation of Zwitterionic Organic-Silica Hybrid Monolithic Capillary Column with an Improved “One-Pot” Approach for Hydrophilic-Interaction Liquid Chromatography (Hilic)',Analytical chemistry,84. 2721-28.
- 4.Liu Yixuan, Chen Yingzhuang, Yang Huihui, Nie Lihua, Yao Shouzhuo. (2013) Cage-Like Silica Nanoparticles-Functionalized Silica Hybrid Monolith for High Performance Capillary Electrochromatography Via “One-Pot” Process’,Journal of Chromatography A,1283. 132-39.
- 5.Colón Héctor, Zhang Xin, Jessica K Murphy, José G Rivera. (2005) and Luis A Colón, ‘Allyl-Functionalized Hybrid Silica Monoliths’,Chemical communications,22. 2826-28.
- 6.Luis A Colon, Li L. (2008) Organo-Silica Hybrid Monolithic Columns for Liquid Chromatography’,Advances. in chromatography.46 (391)
- 7.Lin Xucong, Wang Xiao. (2012) Tingting Zhao, Yanqiong Zheng, Shaofeng Liu, Zenghong Xie, ‘Electroneutral Silica-Based Hybrid Monolith for Hydrophilic Interaction Capillary Electrochromatography’,Journal of Chromatography A,1260. 174-82.
- 8.Andrzejewska Ewa. (2015) Agnieszka Marcinkowska, Dawid Prządka. ‘Multimethacryloxy-Poss as a Crosslinker for Hydrogel Materials',European Polymer Journal,72. 34-49.
- 9.Lin Hui, Ou Junjie, Liu Zhongshan, Wang Hongwei, Dong Jing et al. (2015) Facile Construction of Macroporous Hybrid Monoliths Via Thiol-Methacrylate Michael Addition Click Reaction for Capillary Liquid Chromatography’,Journal of Chromatography A,1379. 34-42.
- 10.Zhu Tao, Kyung Ho Row. (2012) Preparation and Applications of Hybrid Organic–Inorganic Monoliths: A Review’,Journal of separation science,35. 1294-302.
- 11.Ou Junjie, Lin Hui, Zhang Zhenbin, Huang Guang, Dong Jing et al. (2013) . Recent Advances in Preparation and Application of Hybrid Organic‐Silica Monolithic Capillary Columns’,Electrophoresis,34 126-40.
- 12.Wu Minghuo. (2010) Ren’an Wu, Ruibing Li, Hongqiang Qin, Jing Dong, Zhenbin Zhang, Hanfa Zou, ‘Polyhedral Oligomeric Silsesquioxane as a Cross-Linker for Preparation of Inorganic− Organic Hybrid Monolithic Columns’,Analytical chemistry,82. 5447-54.
- 13.Ou Junjie, Zhang Zhenbin, Lin Hui, Dong Jing, Wu Minghuo. (2012) Preparation and Application of Hydrophobic Hybrid Monolithic Columns Containing Polyhedral Oligomeric Silsesquioxanes for Capillary Electrochromatography’,Electrophoresis. 33, 1660-68.
- 14.Ou Junjie, Zhang Zhenbin, Lin Hui, Dong Jing, Zou Hanfa. (2013) Polyhedral Oligomeric Silsesquioxanes as Functional Monomer to Prepare Hybrid Monolithic Columns for Capillary Electrochromatography and Capillary Liquid Chromatography’,Analytica chimica acta,761. 209-16.
- 15.Liu Zhongshan, Ou Junjie, Lin Hui, Wang Hongwei, Dong Jing et al. (2014) Preparation of Polyhedral Oligomeric Silsesquioxane-Based Hybrid Monolith by Ring-Opening Polymerization and Post-Functionalization Via Thiol-Ene Click Reaction’. , Journal of Chromatography A 1342, 70-77.
- 16.Sveca Frantisek. (2010) Porous Polymer Monoliths: Amazingly Wide Variety of Techniques Enabling Their Preparation’. , Journal of Chromatography. A 902-24.
- 17.Gritti Fabrice, Guiochon Georges. (2014) The Rationale for the Optimum Efficiency of Columns Packed with New 1.9μm Fully Porous Titan-C18 Particles-a Detailed Investigation of the Intra-Particle Diffusivity’. , Journal of Chromatography. A 1355, 164-78.
