Evaluation of A Nutrition Feeding Algorithm for Children and Adolescents Undergoing Haematopoietic Stem Cell Transplantation (HSCT)
Abstract
Background
Nutrition support during the acute phase post allogeneic haematopoietic stem cell transplantation (HSCT) is required to optimise short- and long-term outcomes for children. An algorithm was developed and evaluated to assist clinicians to make objective and consistent enteral feeding decisions.
Methods
The algorithm was evaluated on all patients who underwent allogeneic HSCT treatment between November 2017 - February 2019.
Results
Of the 48 patients, 43 had a nasogastric tube (NGT) inserted, of which 36 patients received a hydrolysed peptide-based formula, 5 patients received a whole protein formula only and 2 patients were fed an amino acid-based formula. Parenteral nutrition (PN) was used in 41 of the patients. Eleven did not have an NGT in-situ at the commencement of HSCT. Of the remaining 37 patients, 26 followed the algorithm and 11 patients did not comply. The group of patients who did not follow the algorithm had the longest median length of stay (LOS) of 49 days. Patients receiving only EN had the lowest median LOS of 30 days. The two groups that reported better weight outcomes were those who followed the algorithm and those who were fully EN fed.
Conclusions
Effective use of the HSCT feeding algorithm indicated improved patient outcomes for children undergoing HSCT, with better weight outcomes and reduced LOS. Recommendations to improve the efficacy and compliance of the algorithm include regular education/input to the oncology medical teams to better understand objective thresholds for EN and PN commencement.
Author Contributions
Academic Editor: Sasho Stoleski, Institute of Occupational Health of R. Macedonia, WHO CC and Ga2len CC
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Jodie Bartle
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Allogeneic haematopoietic stem cell transplantation (HSCT) following myeloablative conditioning is a toxic procedure requiring intensive nutrition support. Oral intake during the acute post-HSCT period is decreased due to vomiting, anorexia, mucositis, and diarrhoea. As a result, children who undergo this procedure are high risk for malnutrition1. Malnutrition has been associated with increased risk of infection, transplant related mortality and relapse risk2,3.
Nutrition support is required during the acute phase post HSCT to optimise both short and long term outcomes for children 4, however, despite this being well recognised, strategies to address children’s nutritional needs are inconsistent.
To date, parenteral nutrition (PN) has been considered the method of choice for nutritional support for patients undergoing allogeneic HSCT 3, 5, 6, 7. However, PN-related complications including metabolic/hepatic disorders, central venous catheter sepsis and gut mucosal atrophy remain common, with complication risk shown to increase with longer duration of PN 8.
Enteral nutrition (EN) has been proposed as the first recommended feeding method post HSCT if the gastrointestinal (GI) tract is functional. This helps to maintain intestinal function and integrity, reduces potential translocation and is associated with minimal complications 2, 5, 9. EN has been shown to be safe and effective for feeding patients post HSCT 10, 11. EN is associated with reduced complication rates, improved survival, less acute graft versus host disease (GVHD), faster neutrophil recovery and reduced hospital length of stay (LOS) 2, 5. Financial benefits are also documented 6,11. The choice of formula for enteral provision varies. Many studies report use of polymeric formula throughout HSCT 4, 14, 15, while other reports examining intolerances in severe GI disorders such as intestinal failure suggest extensively hydrolysed formula is better tolerated 16.
Tube feeding tolerance for patients secondary to GI toxicities is a significant concern 12 with challenges for clinicians including the timing of EN initiation, formula choice, and rate of grading. These decisions are mostly dependent on the severity of the GI toxicity and clinician experience. Standardised scales such as the World Health Organization’s Gastrointestinal Toxicity Scale and Common Terminology Criteria for Adverse Events Scales have been developed to classify the severity of GI toxicities to allow for consistent classification of symptoms. Adverse events such as anorexia, vomiting, diarrhoea and mucositis are graded from 1-4 13.
Children undergoing HSCT often have significant GI toxicity with grades of 3/4 mucositis and diarrhoea for prolonged periods. In the absence of clear evidence in HSCT and when significant GI toxicities are expected, we hypothesise that the most likely tolerated feed would be an extensively hydrolysed formula that provides a hydrolysed protein source together with a proportion of medium chain triglycerides (MCT) and a glucose polymer. Hydrolysed proteins that require less time for digestion and absorption are suggested due the decreased transit time associated with gastrointestinal (GI) toxicity 16. As residual lactase and sucrase levels are often compromised and lead to carbohydrate malabsorption and osmotic diarrhoea, glucose polymers may be better tolerated, as they require only gluco-amylases for digestion. A high portion of the fat source as MCT may be better tolerated as it does not require bile-acids for digestion and may be better absorbed and tolerated where significant GI toxicities are evident. Amino acid base formulas should only be used sparingly due to their higher osmolality which may contribute to osmotic diarrhoea. Free amino acids are less likely to promote gastrointestinal recovery 17.
A feeding algorithm for children undergoing HSCT was developed to assist clinicians to make objective decisions on timing of enteral feed initiation, appropriate formula choice and plan for grading up feeds based on objective measures. The algorithm uses standardised gastrointestinal toxicity scales to allow for uniform classification of symptoms to help make consistent objective decisions around appropriate use of enteral feeds and PN during the acute HSCT phase.
The aim of this project was to evaluate the clinical efficacy of the algorithm in different oncology patient groups and diagnoses and its resultant impact on patient clinical outcomes including the effective use of a peptide based enteral feed to optimise enteral nutrition (EN) opportunities during this acute stage of HSCT. Barriers to the HSCT feeding algorithm implementation were also to be identified and documented.
Methods
Study Design and Participants
The HSCT feeding algorithm (Figure 1 and Table 1) has been implemented since November 2017 to guide nutrition support decision making for all children undergoing allogeneic HSCT. All patients aged 0-18 years who underwent allogeneic HSCT treatment were recruited to this prospective study between November 2017 - February 2019. Day 0 (D0) was defined as the day of the stem cell return. Patients were excluded if they died prior to day 30 (D30) post HSCT. Patients with an uncomplicated pre-transplant course were hospitalized 7 to 10 days before transplant whereas children requiring inpatient nutritional rehabilitation or presenting with other medical complications were admitted earlier such as those related to immunodeficiencies or leukaemia treatment related side effects. Pre-transplant conditioning therapy differed according to disease type and patients’ characteristics and was administered 7 to 10 days before transplant. The medical team/staff working within the HSCT unit were provided education regarding the feeding algorithm decision pathway from the unit dietitian. Standard nutritional practice for HSCT patients (Figure 2), remained consistent over this time period, with the addition of the HSCT feeding algorithm to guide recommendations for use of EN and PN based on objective clinical symptoms.
Figure 1.HSCT Feeding Algorithm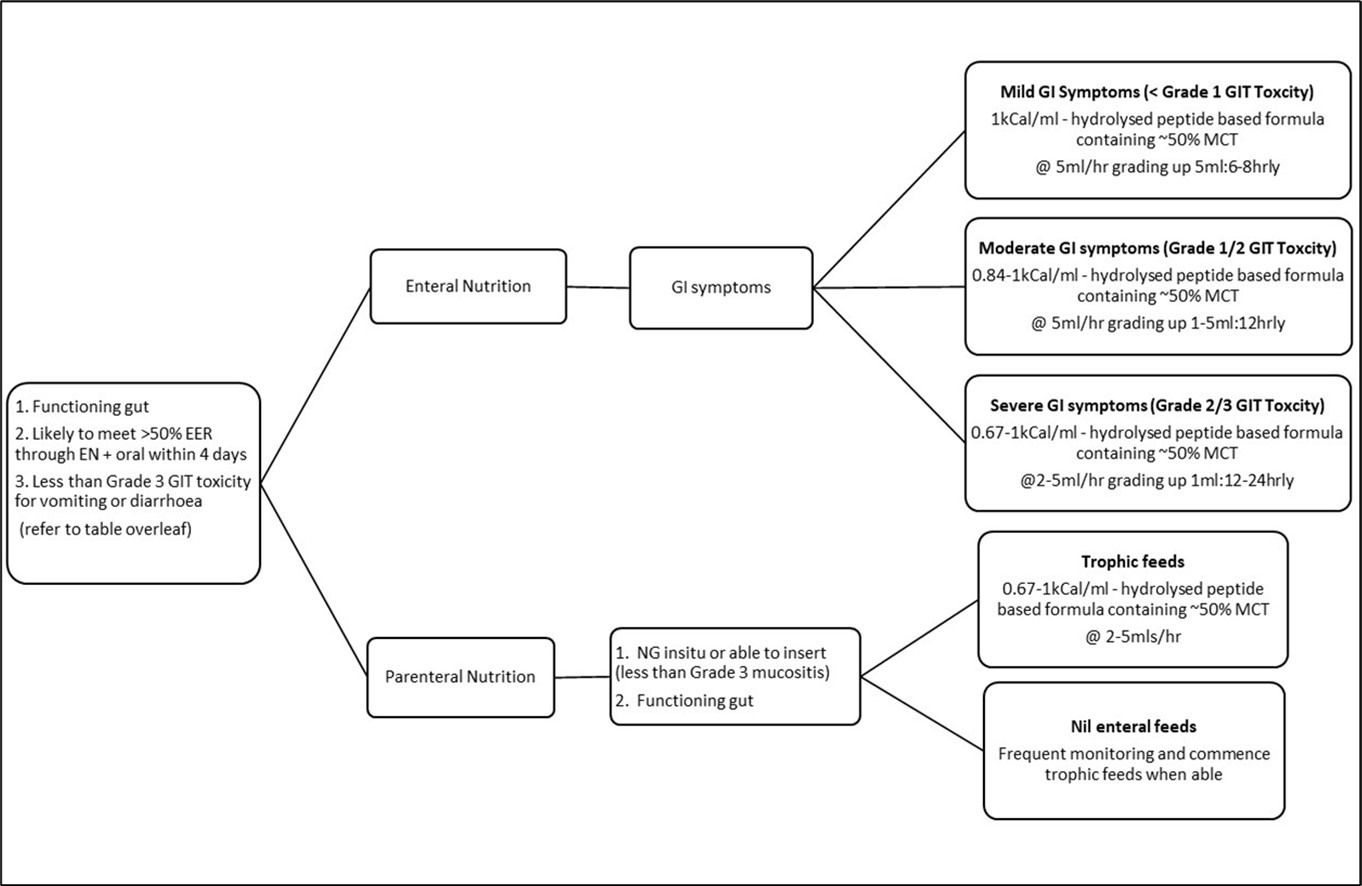
Figure 2.Standard nutrition support processes at RCH
| Adverse event | Grade I | Grade II | Grade III | Grade IV |
| Anorexia | Loss of appetite | Oral intake significantly decreased | Requiring IV fluids | requires enteral / parenteral support |
| Vomiting | 1 : 24hrs | 2-5 : 24hrs | >6 episodes over 24 hours | Requiring parenteral nutrition support or physiologic consequences requiring intensive care; haemodynamic collapse |
| Diarrhoea | 5-10mls/kg/d<4 stools / day | 10-15ml/kg/d4-6 stools / day | >15m/kg/d>7 stools / day | physiologic consequences requiring intensive care; haemodynamic collapse |
| Oral mucositis | Painless ulcers, erythema or mild soreness in the absence of lesions | Painful erythema, oedema, or ulcers, but can eat or swallow | Painful erythema, oedema, or ulcers, preventing swallowing or requiring hydration or enteral / parenteral support | Severe ulceration or requires enteral / parenteral support or prophylactic intubation (or documented aspiration pneumonia) |
Data was collected from the an electronic medical record for all patients from Day 0 (D0) up to Day 100 (D100). Data included clinical diagnosis, transplant type, EN/PN use and anthropometric data. Weight, height and weight-for-length (< 2 years of age) or body mass index (BMI) (≥ 2 years of age) were recorded. The results were plotted on World Health Organization (WHO) growth charts for children < 2 years of age and on Centre for Disease Control (CDC) growth charts for children ≥ 2 years of age and the associated z-scores were calculated. Complications on EN/PN was documented but not specifically reported due to the many confounding factors in interpretation. Compliance with the HSCT feeding algorithm decision pathway was also recorded and barriers to its use identified. Data was collected until each patient was discharged from their acute HSCT admission and then again at D100. Length of stay (LOS) was defined as number of days admitted from D0 until discharge.
Nutritional Support
As part of standard practice, oral intake was encouraged if tolerated using a low bacterial ‘clean diet’ from HSCT admission date or on commencement of conditioning therapy. EN was started when a nasogastric tube (NGT) was inserted electively on D-1 or D0, or earlier if required. When a NGT was in situ, EN was commenced continuously with the rate titrated against oral intake.
From D0, enteral feeds were changed to a hydrolysed peptide-based formula containing ~50% MCT to maximise EN delivery and tolerance (if not in use already). If the NGT dislodged during significant GI symptoms prior to engraftment, then in most instances it was not replaced until the patient had engrafted. The rate of EN was titrated to meet estimated energy requirements and GI tolerance. When EN provided < 50% of energy requirements for 4 consecutive days, PN was commenced and EN was adjusted according to tolerance. If NGT feeds were required pre and post the acute transplant period (before D0 and after D30), then a whole protein formula with energy density of 67kCal (280KJ) – 200kCal (840kJ)/100ml was used depending on the age, energy needs and GI tolerance.
Estimated energy requirements (EER) were calculated using either Nutrient Reference Values (NRV) for children under 3 years or the Schofield equation to measure basal metabolic rate (BMR) x 1.2-1.4 stress/activity factor for the pre and post-acute HSCT period. From D0 to D30, energy requirements were reduced to NRV x 0.8-0.9 and Schofield BMR with no activity factor. This has been the practice based on clinical judgment and experience with reduced energy requirements also evidenced in the literature 18, 19, 20. Protein requirements were estimated to meet the needs of critically ill children ranging from 1.5-3g/kg/d depending on age 21 and fluid targets were calculated using National Health and Medical Research Council guidelines 22.
Data Management and Analysis
Results have been expressed as frequencies and percentages for categorical variables and as means and medians for continuous variables. Statistical analysis were performed for key outcome variables using a variety of tests appropriate to the sample size and distribution of the data including Fisher’s Exact test, Mann-Whitney, Two-sample t-test with unequal variances and a Generalised linear regression model. All analysis was performed using Stata version 16.1 (StataCorp, College Station, TX, USA, 2020).
Results
Between November 2017 - February 2019, 50 HSCT were performed with 48 patients (30 males, 18 females) recruited into the study. Of these, 2/48 underwent 2 transplants (therefore, counted as 2 separate HSCT patients) and 2/48 were excluded due to death prior to D30. The total number of transplants included is n=48. Of those transplanted, 26/48 were for a malignancy, 14/48 for an immunological condition, and 8/48 for a haematology disorder. No patients were transplanted for metabolic conditions during the time of the study. Of these transplants, 17/48 (35%) underwent a matched sibling transplant, 15/48 (31%) had a matched unrelated donor, 13/48 (27%) underwent a haplo-identical transplant and 3/48 (6%) had an unrelated cord donor transplant. Age at transplant ranged from 2 months to 17 years and median LOS was 47 days (range 26-229 days).
Of the 48 patients recruited, 43 (90%) had a NGT inserted and were considered for EN support using a standard nutritional assessment tool and HSCT feeding algorithm. Of these 43, 17 (40%) had a NGT placed prior to their BMT admission, 20 (47%) had an NGT placed on or around D0 and 6 (14%) had an NGT placed post engraftment. Five patients (10%) refused to have an NGT placed. These children were aged between 10-17 years.
All patients required some form of nutritional support during their transplant. Of these, 7/48 (15%) received EN only without PN. The five patients who did not have a NGT inserted received PN only. Of the 7 patients who received EN alone, all met greater than 40% of their EER via EN throughout their admission. A total of 43/48 (90%) patients received some EN during transplant with the average number of days on EN reported as 40 days.
Of the 43 patients enterally fed, 36 (84%) patients received a hydrolysed peptide-based formula at some point during their admission, 5 patients received a whole protein formula only and 2 patients were fed a free amino acid-based formula. Of the five who received a whole protein formula, four commenced EN post engraftment. The one patient who received a whole protein formula throughout the HSCT was established on this formula prior and bowels remained normal throughout the acute phase.
Of the patients who commenced EN, 30/43 (70%) patients required continuation of EN post discharge from acute HSCT admission. Fourteen (33%) had EN weaned prior to discharge, 2 required an extended stay for rehabilitation and 2 died during HSCT (post D30 so still included in study). At D100, 16 (37%) patients remained on EN and 3 (7%) remained on PN/EN.
PN was used in 41/48 (85%) of the patients. Twenty patients were commenced on PN based on the feeding algorithm decision pathway and 11 patients were commenced on PN despite this not being indicated by the feeding algorithm. Eleven patients required nutrition support via PN due to the missed opportunity for NGT placement before or during HSCT. For those receiving PN, average days on PN were 56 days, with one patient who was PN dependant for 237 days.
Of the 48 recruited patients, 11 (23%) were unable to follow the algorithm decision pathway as they did not have an NGT placed during the acute phase of HSCT. Of the remaining 37 patients, 26 (70%) followed the algorithm decision pathway as planned and 11 (30%) patients did not comply. Reasons for patients not following the algorithm decision pathway included pre-emptive commencement of PN without significant GI symptoms (n=6) and failure to grade feed due to GI toxicity concerns (clinician bias) despite not meeting objective criteria of the GI toxicity scale score (n=5).
The impact of the use of the HSCT feeding algorithm on LOS was investigated. This data was not normally distributed with a large skew, hence median (range) days are reported, and a Mann-Whitney test used for analysis (refer to Table 2). The group with the longest median LOS of 49 days were the group of patients who did not follow the algorithm (p=0.378). Those receiving EN only had the lowest median LOS of 30 days. The analysis was repeated excluding the outlier with the large LOS (229 days) but this did not change the results.
Table 2. Comparison of the adherence to the HSCT feeding algorithm by diagnostic groups, HSCT donor type, LOS, weight change and age.| Diagnostic groups | n=48 | Followed algorithm | Algorithm not followed | Excluded as no NGT in-situ | |
| Oncology | 26 | 13 (53%) | 5 (19%) | 8 (31%) | |
| Immunology | 14 | 9 (64%) | 5 (36%) | 0 (0%) | |
| Haematology | 8 | 4 (50%) | 1 (13%) | 3 (38%) | |
| HSCT Donor Types | n=48 | Followed algorithm | Algorithm not followed | Excluded as no NGT not in situ | |
| Matched Sibling | 17 | 9 (53%) | 3 (18%) | 5 (29%) | |
| Matched Unrelated | 15 | 9 (60%) | 2 (13%) | 4 (27%) | |
| Cord | 3 | 1 (33%) | 1 (33%) | 1 (33%) | |
| Haplo identical | 13 | 7 (54%) | 5 (38%) | 1 (8%) | |
| Length of stay, weight and age data comparisons | n=48 | Followed algorithm (n=26) | Algorithm not followed (n=11) | Excluded as no NGT not in situ(n=11) | EN only(n=7) |
| Median LOS in days (range) | 41 (26-229) | 49 (33-70) | 48 (27-85) | 30 (26-56) | |
| Mean change in weight z score (D0 to D100) | 0.17 | -0.12 | -0.1 | 0.1 | |
| Mean age in years | 7.5 | 6.3 | 14 | 9 | |
| Sex | |||||
| Males | 17 | 7 | 6 | ||
| Females | 9 | 4 | 5 | ||
The impact of the use of the HSCT feeding algorithm decision pathway on weight outcomes were reported as weight change in z scores between D0 and D100. The two groups that reported better weight outcomes during the HSCT were those who followed the algorithm and those who were fully EN fed without the use of PN (Table 2). An average change in weight for those who followed the algorithm was 0.28 standard deviations higher than for those who did not (95% CI -0.19, 0.76; p=0.228).
The association of the use of the algorithm with patient age was also investigated. Using a generalised linear regression model there was a 0.7% (95% CI -2.2%, 3.5%; p=0.648) average increase in adherence for each year of age. The patient group that refused NGT placement were generally older with a mean age of 14 years There was no apparent significant gender difference associated with the compliance of the algorithm (p=>0.99) (Table 2).
There were no observed differences between diagnostic groups (p=0.891) or HSCT donor type (p=0.521) based on whether the algorithm was followed. Table 2 shows the proportion from each group whose nutrition management was compliant with the algorithm decision pathway. All immunology patients had an NGT placed at some stage during their BMT and haematology patients had the highest proportion of children that refused to have an NGT placed. While all cord and haplo HSCT patients had an NGT placed at some point during their HSCT, 1 patient in each group did not have an NGT insitu during the acute phase so had to be excluded.
Discussion
The HSCT feeding algorithm decision pathway for children undergoing HSCT at was implemented and evaluated. The algorithm was designed to guide objective decision making for nutrition support in this patient group. Effective use of the algorithm indicated improved patient outcomes with better weight outcomes and reduced LOS. Although these differences were not statistically significant this is likely a reflection of the small sample size and limited power of the study to detect these differences. Barriers to its use have been identified as patient age and clinician bias which resulted in missed opportunity for use of EN and inappropriate use of PN where not clinically indicated for some patients. This may have contributed to increased LOS and associated costs, yet no additional benefit to patient nutritional outcomes to warrant this.
Prior to implementation of the algorithm, a study conducted at the same centre between 2014 -2017, collected data on 31 children undergoing HSCT. This study reported detrimental weight z scores (-0.23) and higher median LOS (67 days) which is consistent to that reported in our cohort of children who did not follow the algorithm compared to the group that did and further evidence of the efficacy of the algorithm 27.
The use of PN when not clinically indicated was the main barrier identified to algorithm compliance. This decision was based on clinician opinions and attitude towards EN at the time of patient review and not objective measures. This was most commonly reported as pre-emptive concerns about worsening gastrointestinal symptoms and lack of confidence in grading up feeds. Information was collected on 48 patients over a 16-month period. Over this period the medical and dietetic treatment teams changed which may have contributed to inconsistent decision making. This further supports the need for the use of an evidence-based standardised feeding algorithm decision pathway to guide the clinician on objective decisions for grading up of feeds rather than individual opinion.
Several patients in the cohort could not follow the algorithm as they refused to have an NGT placed and relied on PN support despite having a functional gut. Refusal of NGT placement in these children not only has cost implications due to the PN cost and increased LOS, but also a risk to patient outcomes including increased risk of infection, liver abnormalities and gastro-intestinal mucosal health 23. Furthermore, EN has been associated with better survival, less acute GVHD and faster neutrophil recovery 2. This group of children were on average 14 years of age. There is limited literature available that evaluates adolescent perceptions and attitudes towards NGT support during their oncology treatment. In a recent review, the perspectives of parent, patient and health professional were reported pre- and post-placement of an NGT in children <18 years. The main issues this review documented for pre-tube insertion included concerns about the child’s physical appearance, invasiveness of the NGT and perceived tube discomfort. Post-NGT insertion the reported perceptions were more positive and included opportunity for weight gain, better nutritional intake, less worry/pressure to eat. Essentially, the parent and patient negative views changed once NGT was inserted and the benefits of NGT feeding were realised 24. Increased support and education with patients/families is recommended to ensure realistic expectations and positive patient experience and outcomes.
A Children’s Oncology Group (COG) study examined the nutrition support standards of practice at 125 member sites and showed that enteral feeding was not consistently offered as the first method of nutrition support to patients. Variability in the use of enteral and parental nutrition was dependent on clinician, nurse and dietitian viewpoint and historical practice rather than following best practice guidelines 25.
Effective decision making and acceptance of NGT placement can be facilitated by tools such as a HSCT feeding algorithm to allow unbiased and balanced information provision to guide the best clinical care for the patient. Education and communication for the oncology team around these standardised algorithms may help facilitate objective decisions around NGT support. In addition, often these practices need to be initiated early in a child’s treatment as the success of EN during HSCT can be dependent on a child’s nutrition support experiences throughout their treatment 6.
Due to well documented gaps in implementing evidence-based feeding practices, nutrition support algorithms have been developed to ensure patients receive nutrition care based on the available scientific evidence, with the aim to improve the patient’s nutritional outcomes. These algorithms provide specific steps in the nutrition support decision making process based on patient condition and objective assessment 26. While nutrition support algorithms to guide feeding in oncology patients have been shown to reduce days on PN and facilitate enteral feeding, the practices require ongoing reinforcement and education to clinicians and support from the multidisciplinary team, especially dietitians, nurses and physicians 6. A recommendation for ongoing compliance with the HSCT feeding algorithm includes frequent education sessions for rotating medical teams and nurses.
Ninety percent of patients received EN at some stage during their HSCT. This is a positive outcome as even trophic feeds have been shown to maintain intestinal function and integrity, reduce potential translocation, reduce HSCT complication rates, promote better survival, reduce risk of GVHD, facilitate faster neutrophil recovery and reduce hospital LOS 2, 5, 9, 23. It was also observed that patients who didn’t require any PN or who followed the algorithm had the shortest LOS, and conversely those who didn’t follow the algorithm or refused to have an NGT had the longest LOS. A contributing factor to this finding is because discharge could still be facilitated with home EN support if oral intake was still inadequate, which was not possible with patients on PN despite being medically ready for discharge.
There were no apparent differences in adherence to the HSCT feeding algorithm when comparing diagnosis for HSCT, donor type and sex. The algorithm has been designed using objective criteria of GI clinical tolerance symptoms independent of diagnosis, HSCT donor type and age, and hence has the advantage that it can be used across all types of HSCT condition regimens. Barriers to effective use of the algorithm have been discussed and fall into two main categories. Firstly, being no NGT placement to provide EN and secondly inappropriate use of PN. Opportunities to strengthen relationships with key stakeholders such as the PN team and gastroenterology to help advocate for algorithm compliance may help to overcome future barriers to its use in addition to frequent education within the oncology multi-disciplinary team.
A limitation to the study was the small sample size, however, every patient was recruited into the study and inclusive of anyone who was admitted for HSCT between November 2017 and February 2019 so there is no bias with selection criteria. Therefore, a larger multi-centre study would be a future recommendation to confirm results of this preliminary study.
In addition, other limitations to the study included the potential accuracy of multiple clinicians measuring, reporting, documenting and interpreting the gastrointestinal symptoms and accurate recording and reporting of oral intake. The algorithm suggests a criteria of greater than 50% of EER from EN and oral intake over 4 days to guide decisions regarding nutritional management. Although this may be difficult to estimate daily, all patients at some stage for a period of greater than 4 days were estimated to have less than 20% of their EER from EN or oral intake. Strengths to the study included a consistent team of Dietitian’s for robust data collection and a prospective study design. The use of an electronic medical record was also a major advantage.
Conclusion
Effective use of the HSCT feeding algorithm decision pathway indicated improved patient outcomes for children undergoing HSCT, with better weight outcomes and reduced LOS. Barriers to its use were identified. Recommendations to improve the efficacy of the feeding algorithm decision pathway include regular education and input to the oncology medical teams to better understand objective thresholds for EN and PN commencement.
References
- 1.AMS El-Ghammaz, Matoug R B, Elzimaity M, Mostafa N. (2017) Nutritional status of allogeneic hematopoietic stem cell transplantation recipients: influencing risk factors and impact on survival. Supportive Care in Cancer. 25(10), 3085-3093.
- 2.Baumgartner A, Bargetzi M, Bargetzi A. (2017) Nutritional support practices in hematopoietic stem cell transplantation centers: A nationwide comparison. Nutrition. 35, 43-50.
- 3.Espinoza M, Perelli J, Olmos R, Bertin P, Jara V et al. (2016) Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Revista brasileira de hematologia e hemoterapia. 38(1), 7-14.
- 4.Andersen S, Kennedy G, Banks M. (2015) A randomised controlled comparison of enteral versus parenteral nutritional support post allogeneic haematopoietic cell transplantation. Clinical nutrition ESPEN. 10(3), 102-106.
- 5.Azarnoush S, Bruno B, Beghin L. (2012) Enteral nutrition: a first option for nutritional support of children following allo-SCT? Bone marrow transplantation. 47(9), 1191.
- 6.Steele C, Salazar A, Rypkema L. (2016) Utilization of a nutrition support algorithm reduces unnecessary parenteral nutrition use in pediatric oncology inpatients. , Journal of the Academy of Nutrition 116(8), 1235-1238.
- 7.Cohen J, Maurice L.Adequacy of nutritional support in pediatric blood and marrow transplantation. , Journal of Pediatric Oncology Nursing 27(1), 40-47.
- 8.Mantegazza C, Landy N, Zuccotti G, Köglmeier J. (2018) Indications and complications of inpatient parenteral nutrition prescribed to children in a large tertiary referral hospital. Italian journal of pediatrics. 44-1.
- 9.Bicakli D H, Yilmaz M C, Aksoylar S, Kantar M, Cetingul N et al. (2012) Enteral nutrition is feasible in pediatric stem cell transplantation patients. Pediatric blood & cancer. 59(7), 1327-1329.
- 10.Seguy D, Berthon C, Micol J-B. (2006) Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 82(6), 835-839.
- 11.Hopman G, Pena E, S Le Cessie, M Van Weel, Vossen J et al. (2003) Tube feeding and bone marrow transplantation. Medical and Pediatric Oncology: The Official Journal of SIOP—International Society of Pediatric Oncology (Societé Internationale d'Oncologie Pédiatrique. 40(6), 375-379.
- 12.Garófolo A. (2012) Enteral nutrition during bone marrow transplantation in patients with pediatric cancer: a prospective cohort study. Sao Paulo Medical Journal. 130(3), 159-166.
- 14.Seguy D, Duhamel A, Rejeb M B. (2012) Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 94(3), 287-294.
- 15.Gonzales F, Bruno B, Fuentes M A. (2018) Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clinical nutrition. 37(6), 2113-2121.
- 16.D’Antiga L, Goulet O. (2013) Intestinal failure in children: the European view. Journal of pediatric gastroenterology and nutrition. 56(2), 118-126.
- 17.Lapillonne A, Matar M, Adleff A, Chbihi M, Kermorvant-Duchemin E et al. (2016) Use of extensively hydrolysed formula for refeeding neonates postnecrotising enterocolitis: a nationwide survey-based, cross-sectional study. BMJ open. 6(7), 008613.
- 18.Ringwald-Smith K, Heslop H, Krance R. (2002) Energy expenditure in children undergoing hematopoietic stem cell transplantation. Bone marrow transplantation. 30(2), 125.
- 19.Sharma T S, Bechard L J, Feldman H A. (2011) Effect of titrated parenteral nutrition on body composition after allogeneic hematopoietic stem cell transplantation in children: a double-blind, randomized, multicenter trial. The American journal of clinical nutrition. 95(2), 342-351.
- 20.Duro D, Bechard L J, Feldman H A. (2008) Weekly measurements accurately represent trends in resting energy expenditure in children undergoing hematopoietic stem cell transplantation. , Journal of Parenteral and Enteral 32(4), 427-432.
- 21.Mehta N M, Compher C, ABo Directors. (2009) ASPEN clinical guidelines: nutrition support of the critically ill child. , Journal of Parenteral and Enteral 33(3), 260-276.
- 22.Australian‐Government. (2006) Nutrient reference values for Australia and New Zealand including recommended dietary intakes. In: Commonwealth of Australia Canberra;.
- 23.Ladas E J, Sacks N, Meacham L. (2005) A multidisciplinary review of nutrition considerations in the pediatric oncology population: a perspective from children's oncology group. Nutrition in clinical practice. 20(4), 377-393.
- 24.Cohen J, Wakefield C E, Tapsell L C, Walton K, Cohn R J. (2017) Parent, patient and health professional perspectives regarding enteral nutrition in paediatric oncology. , Nutrition & dietetics 74(5), 476-487.
- 25.Ladas E J, Sacks N, Brophy P, Rogers P C. (2006) Standards of nutritional care in pediatric oncology: results from a nationwide survey on the standards of practice in pediatric oncology. A Children's Oncology Group study. Pediatric blood & cancer. 46(3), 339-344.
