Abstract
Natural selection is a buzzword used to describe the main driving force of evolution. Its creative role is believed to be based on: a) an unlimited variety of organisms caused by hereditary variation and b) a direct connection between hereditary changes and their phenotypic expression. These are the two requirements that can lead to the genetically based changing modalities of characters through “iterations” of natural selection in the series of successive generations. Are these two requirements fulfilled in the nature, however? The present study focuses on the analysis of these two “foundation stones” of natural selection. Firstly, hereditary variation is shown to be essentially non-homogenous. New hereditary characteristics of individuals fall onto a narrow “strip of land” in the sea of potential possibilities. Secondly, the consequences of changes in the genotype of an organism are involved into a system of hierarchical multiple compensation, from the molecular to the biocenotic level. In a way, the signal of hereditary change passes through a series of “system filters” at epigenetic, ontogenetic, physiological, behavioural, populational and biocenotic level. Each filter is represented by multiple feedbacks maintaining the integrity of systems at each level and at all the hierarchical levels taken together. It is in these “system filters” the adaptive nature of characters is formed representing the every individual as a subject to the Law of Multilevel Self-Organization. The emerging understanding of this provides a strong reason to change the evolutionary paradigm from the mainly selectogenetic to the mainly orthogenetic one.
Author Contributions
Academic Editor: Zhencheng Xing, PhD Student, School of Business, Hohai University, China.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Andrey I. Granovitch
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Natural selection, laconically expressed by Spenser and Darwin as “…the survival of the fittest”23, 115, has become a key concept in the interpretation of microevolution events. In various forms, it has been used to explain: adaptive shifts in the modality of character expression under changing environmental conditions (directional selection); maintenance of optimal values of characters in stable environments (stabilizing selection); formation of bimodal frequency distributions followed by the divergence of forms in situations when several optimums are present in the environment (disruptive selection); formation of adaptations directly associated only with the reproductive success during sexual reproduction (sexual selection). The idea of natural selection is the fundamental component of population genetics and generally of the 20th century evolutionary synthesis24, 30, 67, 72, 129. Natural selection underlies generally accepted speciation models 67.
However, in recent years, the notion of NS as an universal mechanism of microevolution has raised more and more questions. There are at least three directions of a critical rethinking of NS. First is a discussion about considering NS as a putative evolutionary mechanism from the point of view of a new mechanistic philosophy 6, 45, 81, 114. Second is expanded understanding of phenotypic plasticity and transgenerational inheritance 25, 62, 64, 93, 127, 130 that dramatically change the relationship between mechanisms of phenotypic plasticity and genotypic adaptations. The last in its turn can change the evolutionary paradigm to the “extended evolutionary synthesis”. Third is growing attention to the mechanisms of self-organization of molecular complexes, cell organelles, cells and multicellular organisms 49, 63, 69, 82, 83. From this point of view, NS is much more restricted than it was previously assumed. It can operates only to form specific conditions for the implementation of self-organization processes. Thus, generally, the last decades again put the notion of NS in the discussion plane and my work represents one more step in this direction.
Within the framework of the modern evolutionary synthesis, studies of the intensity of natural selection are based on the assessment of “fitness”, the degree of reproductive input of an individual into the next generation 4. The idea of “reproductive success”, a greater contribution of an individual into the gene pool of the next generation as compared with the carriers of other genotypes, is central here. It is unimportant whether it is achieved through differential mortality (the survival of the fittest) or in some other way. What is important is that individuals carrying a certain genotype in the series of successive generations (“iterations” of the selection) change the representation of this genotype in the population*.
*Note:, the notion of selection should not be expanded to mean the universal synthesis of the oppositions of “prohibition and permission” (see 77, p. 252). Such an expansion makes it impossible to disentangle the processes of structural self-organization at various levels and the processes ruled by the mechanism of selectogenesis and, thus, to distinguish selectogenetic and orthogenetic evolutionary mechanisms.
Can we prove that different individuals in the population contribute unequally into the next generation? Indeed, we can. That is what all studies of the reproductive structure and the dispersion of fertility indices in populations point to. Many of the individuals simply do not live long enough to reach the reproductive stage. The dispersion of individuals within populations as to the numbers of surviving offspring is always high 19, 21, 36, 84, 92. The length of the pre-reproductive phase varies 38, and the reproductive period itself may be longer or shorter in different individuals in the same population 14, 20, 29, 74. We may, therefore, be fairly sure that the contribution of individuals into the gene pool of the next generation is unequal. Whether this unequal contribution is connected with evolution, a vector process involving series consisting of hundreds and thousands generations is, however, far from being evident. Such a connection, if any, has to be separately proved. It may well turn out that we observe mere stochastic or cyclic populational-genetic processes 1 rather than changes at the evolutionary scale allegedly caused by “natural selection”.
So, the differential contribution of individuals into the gene pool of the next generation as such does not prove that the mechanism of natural selection is at work. This begs the question: can natural selection be considered as the main driving force of evolution? This is the key issue addressed in the present study. To solve it, I will briefly characterize the primary material of natural selection, that is, hereditary variation (section 2, Potential material for natural selection). Then I will analyse, in a series of hierarchical systems, how probable is the formation of a correlation between variations of the genotype and the reproductive parameters of an individual (section 3, System filters…). If found, a distinct correlation of this kind would provide compelling proof of the effectiveness of natural selection as an evolutionary factor.
Potential Material for natural selection
Stating the Question
Natural selection acts upon the entire range of hereditary variation in a given population. There are, however, certain requirements to the nature of this variation. If these requirements are met, we can consider natural selection as an important mechanism of changing the modality of the genotypic composition of the population (to be entirely sure about its effectiveness, one should also analyse the importance of “system filters” – see section 3).
There are two major requirements. Firstly, the variation should be to more or less “homogenous” (or isotropic 53). This means that it should be characterized as unlimited, potentially representing a broad range of variants. The manifestations of hereditary deviations should be distributed more or less evenly across the axes of characters’ variation. The second requirement is as follows: the primary act of hereditary variation should not depend on the need in the act and the nature of this need*.
*Note: In essence, these requirements have been formulated by Meyen and Chaikovsky 80, p. 12, paragraphs 5, 6 and 9 in their review of the ideas of A.A. Lyubishchev concerning selectogenesis.
If both these requirements are met, we may be fairly sure that the subsequent changes in the frequencies of characters indeed can be, in principle, be formed by natural selection. On the other hand, if these requirements are not met, we should rethink the leading role of natural selection in microevolutionary events.
For instance, the “non-homogeneity” of the character space (the first requirement) — in other words, canalized nature of hereditary variation, considerable differences in the frequency of manifestation of the hereditary variants, the discreteness of forms in the hereditary variants — testifies to the importance of the “construction-determined” evolutionary mechanisms and to the prevalence of orthogenesis (Box 1). The greater “non-homogeneity” is observed, the more important orthogenesis is (Figure 1). The role of natural selection is reduced to eliminating non-viable variants and the work within the range of the origin of forms, which islimited and predetermined by the variation 8, 12, 17, 53, 70, 79, 85.
| Box 1 Three types of evolutionary concepts.Indirect adaptogenesis, syn.selectogenesis(term 12, 70), from Latin selectio — choice, selection; syn.tychogenesis(term90, p. 813),from Greek týche — luck, fortune. Heterogeneity of organisms in respect of reproductive characters — random, unlimited and independent of the environment — is the cause of differential input of individuals into the next generations. The major mechanism of transformation is selection of the “fittest”, or natural selection. Cumulatively, in the series of generations, it results in adaptive changes of the modal species characteristics. The environment determined the direction of selection, and therefore the model is ectogenetic: it admits transformism under the influence of external factors. The model is also idiographic, as it focuses on the uniqueness of transformation acts and their probabilistic character. The ideas of indirect adaptogenesis underlie the “modern evolutionary synthesis” of the 20th century. Direct adaptogenesis.The impact of the environment results in adequate changes in the morpho-functional inheritable characteristics of the organism. In this way, adaptive changes of organisms are not mediated by selection but form directly under the influence of the environment in a series of generations. This means that the model admits the existence of ectogenesis, transformation under the influence of external factors. The model is idiographic: each transformation act, being determined by a unique combination of the environmental conditions, is considered as unique. The mechanism of direct adaptogenesis underlies the ideas of various Lamarckian evolutionary hypotheses. Structural transformism, syn. orthogenesis (term 40), from Greek ὀρθός — straight.The model considers internal patterns of morpho-functional organisation of living systems as the driving force of transformation. Its logic can be characterized as “self-assembly” (rather than selection!) of increasingly complex systems. Therefore, it is the only model that consistently admits the existence of autogenesis: the change based on internal patterns of the organisms’ structure. According to this model, variation of organisms is strictly directional rather than random. The model is nomothetic: it searches for strict laws of evolutionary transformations and envisages the possibility of predictive interpretations. On the whole, it admits the existence of internal directionality of the evolutionary process, in other words, orthogenesis. The main problem of orthogenetic interpretations of evolution is associated with the explanation of the formation of adaptations in the course of evolution, that is, their correspondence to the environment 70. |
Failure to fulfil the second requirement spells the acknowledgement of direct adaptogenesis (see Box 1. Three types of evolutionary concepts). Direct adaptogenesis occurs when the impact of the environment on the individual is accompanied by the formation of the hereditary variants of the progeny that are, in general, consistent with the impact (inheritance of acquired characters). This situation is essentially different from that envisaged by the natural selection model, where adaptations are formed indirectly, by selection from the available variants (indirect adaptogenesis).
What are, in fact, the manifestations of the hereditary variation? Are we in the position to say whether the two requirements are fulfilled and to provide the rationale of the effectiveness of natural selection?
Arguments in Favour of Macro-Evolutionary Approaches to the Assessment of Variation Structure
The most general approach to the assessment of the “homogeneity” of the character space is the construction of potentially possible (mathematically calculated) spaces of certain characters. Within this approach, the calculated “morphological space” (“morphospace”) of the potential variation of a character is compared with that actually implemented in a certain species of organisms, group of species or group of organisms united into taxonomical categories of a higher rank. In all these cases it has been shown that only a minor proportion of the potential diversity is actually implemented in the nature 18, 75, 78, 100, 101, 102, 113, 118, 119, 121. This discrepancy between the potential and the actual diversity may be an argument in favour of essential structural constraints of the origin of forms(that is, the primary “non-homogeneity” of variation). At the same time, this assessment is not necessarily incompatible with a different viewpoint: the initial variation is reasonably “homogenous” and the subsequent non-homogeneity is the result of the mechanism of differential mortality, that is, natural selection.
The analysis of hereditary variation of characters in closely related species allows one to make more definite conclusions. The range of variation and particular characters turn out to be rather conservative in species of the same genus and even the same family. Manifestation of the same range of forms in the variation of different species may concern both particular morphological characters and complexes of correlated characters representing certain “morphotypes”. This phenomenon is best described by Vavilov’s law: the law of homologous series of hereditary variation 123. It emphasizes, firstly, the similarity of series of variation in closely related species and genera (the similarity being directly proportional to the relatedness of the taxa under comparison) and, secondly, the similarity of cycles of variation in subordinate taxa within the category of a higher rank (such as a family). Noteworthy, discussing the formation of such parallelisms of variation, Vavilov admitted the possibility of a similar direction of the mutation process in closely related species (see 123). Homologous series reflect the nomothetic nature of variation: it is not stochastic but subject to general rules. Such ideas are incompatible with the initial hypothesis about the “homogeneity” of variation. Nomothetic nature of variation is discussed in detail by Vasil’yev and Vasil’yeva 122 and widely discussed in modern literature (see, for example 8, 53, 85).
The nomothetic nature of variation at the level of macrotaxa is strongly supported by paleontological data on the evolutionary formation of large taxonomic groups of organisms: archaeocyathids 104, arthropods 95,96, mammals 117, echinoderms 105 and birds 71. Limitations applying to the origin of forms and numerous parallelisms indicate that Vavilov’s law of homologous series is expressed at the scale of evolution of marcotaxa 106. Therefore, the “non-homogeneity” of variation may play a pivotal role in directed character of the evolution.
Ideas about limitations of variation find an even more general expression in the concept of repeating polymorphic sets 78,113. The emphasis here is on the distributions of the modalities of separate characters (rather than character sets as in Vavilov’s law) and their similarity (“isomorphism” according to 78) in different taxa. Moreover, the notion of repeating polymorphic sets falls outside the scope of phylogenetic homology. Their repetition “…can be observed in obviously non-homologous parts …” (78, p.165). This is equivalent to an outright acceptance of the existence of some laws of variation and origin of forms that are not subject to “phyletic” relationships. From this viewpoint, the potential role of natural selection in origin of forms is reduced to secondary corrections.
An important argument in favour of a considerable “non-homogeneity” of the space of hereditary variation is the wide distribution of agamic, and parthenogenetic species among protists and multicellular organisms 39, 44, 107. Their morpho-functional distinctness is not associated with any special mechanisms uniting their gene pool and at the same time isolating it from other genetic entities. The very existence of distinct species in such organisms is an evidence of the discreteness of the origin of forms and its canalized nature.
Another argument jeopardizing the hypothesis about the “homogeneity” of the space of hereditary variation is provided by the existence of numerous so-called cryptic species. These are genetically separate groups endowed with all the prerequisites for morpho-functional divergence on the basis of their genetic separateness. The scale of genetic differences in such groups may vary; in fact, they are often more than sufficient for these species to be considered as reliable “biological species” 16. However, despite the genetic divergence, the representatives of these groups are characterized by a conservative structure and an extreme paucity of morpho-anatomical differences. Cryptic species occur in a variety of taxonomic groups of eukaryotes, from protists to multicellular animals 44, for a review of multicellular animals see 120. The importance of “non-visual signals” for the establishment of reproductive isolation and the stabilizing selection for a certain morphotype have been used to explain the evolutionary formation of such complexes of species 16. However, the problem of their morphological conservatism remains. Actually, under conditions of an acquired (no matter how) reproductive isolation there should be an extremely effective mechanism of maintaining the conservativeness of morphological characters (stabilizing selection?), also explaining an impressive intraspecific plasticity in some species (directional selection at the same time ?) 48, 97, 108 (“anti-cryptic selection”?) 16. The explanations of this phenomenon from the point of view of limitations in the origin of forms, the “non-homogeneity” of character space, are more parsimonious.
Variation at Early Ontogenetic Stages
Several attempts have been made to assess directly variation in organisms at the early stages of the ontogenesis (that is, before the time when variants may be eliminated by natural selection). An example of such an analysis is the study of variation of postcranial skeleton (spinal column, sacrum, pectoral and pelvic girdle, limbs) of tailless amphibians 58, 59, 60. The results indicate that а) the range of actual variation is considerably more narrow than that of the theoretically calculated potential variation; it is represented by series of discrete variants; b) there exist “prohibited” variants, which are never implemented, and the set of such variants is different in different species; c) under extreme developmental conditions the proportion of anomalous individuals increases but the general set of variants remains the same. Therefore, both the “non-homogeneity” of the character space and the influence of environmental conditions on the frequency of actual anomalous variants is observed.
In many instances hereditary variation is characterized by a considerable discreteness of the manifestation of the entire complexes of characters in an organism 2, 17. A theoretical basis of these observations can be found in the ideas about correlative connections in an organism and the canalization of the ontogenetic ways 109,125.
Consistent Formation of Variation at the Molecular Level
Selectogenetic model of evolution is based on the idea that potential hereditary changes are in principle unlimited and multidirectional. However, ideas about systemic organization of genomes 34, 51, 112, 128 leave little space for interpreting molecular mechanisms underlying hereditary variation as stochastic processes. Failures of matrix processing in prokaryotes and eukaryotes are mitigated by an array of reparation systems 26, 50, 98. These systems, in turn, can be fine-tuned and regulated, resulting in regulated changes in the level of mutational variation 31,94. The activity of horizontal transfer in prokaryotes is associated with the systems of DNA reparation. Activation of stress-induced mutagenesis leads to mobilization of conjugative elements, which are used as vectors of genes responsible for antibiotic resistance 7,43.
Much of hereditary variation is associated with the transfer of mobile genetic elements in the genome of different species 3, 28, 32, 54, 56, 73. Their transfer is often directional, sometimes involves large numbers of elements and may result in quite definite hereditary changes 103.
An impressive array of epigenetic mechanisms involved into the formation of variation provides an insight into the molecular basis of hereditary changes. At the same time, the work of epigenetic systems is essentially non-stochastic. It is a reflection of a system of negative feedbacks in the genome in the narrow sense of the word (that is, a set of species-specific sequences of DNA bases) and other molecular components of the cell associated with the regulation of the protein-synthesis apparatus, the dynamic chromatin structure, “protein” heredity and “small RNA” systems (see a series of reviews in Epigenetics 27).
Ideas about the systemic organization of the genetic-epigenetic mechanisms are reflected in the conclusion that the reconstructions of the hereditary apparatus comply to the external influences. It is not so much the matter of correlations between the impact of the environment and the frequency of hereditary changes (see above, regulation of reparation systems) but rather the compliance of molecular reconstructions of the genetic-epigenetic system to this impact. Examples of this compliance are a higher level of mutations in those bacterial sites whose products are involved in the potential compensation of the external influence 41, 42; amplification of functional areas of genome associated with the resistance to chemicals in protists 15; specific inserts into genome CRISPR cassettes in bacteria ensuring resistance to bacteriophages 55, 56, 91 similar mechanisms in eukaryotes associated with the activity of piRNA and siRNA 3,52; paramutations, whose manifestation also seems to be associated with the mechanisms of RNA interference 47. All this prompts one to revisit the idea of the direct inheritance of adaptive molecular changes at the epigenetic and even the genome level in the light of molecular mechanisms 34, 55, 56, 65.
Nature of Variation: Summing Up
It has been shown that at least in some cases variation is directional and its directionality is sometimes induced (by environmental impacts). Recalling the two major requirements to hereditary variation, we can make the following conclusion: observations on intra- and interspecies variation point to a considerable “non-homogeneity” of the variation implemented in the character space. The fact that this non-homogeneity, rather than being a post hoc result of differential mortality, is associated with the character of variation is proved by: a) the presence of general trends, variation series; b) direct assessments of variation at early stages of ontogenesis; c) the analysis of molecular mechanisms underlying hereditary variation; d) the possibility of direct observation (in case of laboratory models for the study of variation). Moreover, it can be considered as a fact that the frequency of hereditary reconstructions increases under the influence of external factors. There are also indications of correlations between the character of the impacting factor and the type of hereditary reconstructions. The structure of variation is “non-homogenous” in its manifestations at the level of organisms and considerably “non-homogenous” in manifestations of hereditary changes at the level of genome and the associated epigenetic processes.
To conclude, at the imaginary axis of the “homogeneity” of hereditary variation (from entirely random to strictly canalized) its modal values are evidently confined to the area of consistent expression of characters. This entails a conclusion that orthogenetic mechanisms of the formation of variation predominate while natural selection plays a secondary role. Its importance diminishes even further in the context of data indicating that hereditary variation may conform to certain environmental impacts.
Further analysis would focus on the possibility of natural selection “using” hereditary variation. Let us imagine a change in the genotype of an individual as a signal which should correlate with the ability of this individual to make a greater or a smaller contribution into the gene pool of the next generation. The character of variation is no longer of interest to us; the emphasis will be on the consequences of its indisputable presence.
System Filters and the Effectiveness of “Hereditary Signal” for Natural Selection.
Variation determines the range of the morpho-functional variants of individuals of a given population or species. We will consider each change in the genotype structure as a “signal”, which is to be reflected in the characteristics of the individual (regardless of the nature of the inherited reconstructions, be they gene mutations, chromosomal reconstructions, changes associated with ploidy, reconstructions in the course of crossing-over or recombination variation of combinations during sexual process). In the long run, these characteristics should be reflected in the differential contribution of individuals into the gene pool of the next generation. From now on, we will ignore the changes that occur in the genotype but are not manifested, by definition, in the morphological and functional characters of an individual (for instance, synonymous base substitutions).
The “hereditary signal” becomes involved in interactions representing various hierarchical levels of the systemic organization of living nature. Starting at the suborganismic level, the interactions ascend to the biocenotic one. Each level is characterized by specific mechanisms of maintaining systemic integrity; the systemic integrity of the next level comprises all subordinate mechanisms but cannot be reduced to their sum. These holistic notions are reflected in the concept of emergence and can be aphoristically expressed as “the whole is bigger than the sum of its parts“ (68; see 22 for historical context and development of the notions of emergence into the Synergism Hypothesis).
Hereafter the term “system filter” will be used to denote the system of feedbacks and compensatory regulations at each of the hierarchical systemic levels and the passage of the hereditary “signal” through a series of “system filters” will be considered. Special attention will be given to the stability of the “signal” since only stable passage through the filters may result in the implementation of the natural selection mechanism.
A. Filter no. 1. Epigenetic Regulation.
The change in the genotype structure (the “hereditary signal”) should be considered in the context of a system of dynamic molecular interactions including the regulation of genes’ activity by transcription factors, RNA interference, alternative splicing, DNA methylation and histone acetylation, mobility control of mobile genetic elements. In general, all these mechanisms belong to the system of epigenetic regulation of the genetic networks 27,126. The consequences of structural changes in the genome would thus be reflected in multiple compensatory reactions of the epigenetic system of the cell. The systemic character of the complex of these reactions is expressed in the maintenance of a stable whole (the entire molecular-genetic system of the cell) on the basis of the compensatory reactions of its elements (changes of the state of functional blocks of this system).
B. Filter no. 2. Ontogenetic regulation (integrity of the morphoprocess).
Transformed by the epigenetic system of the cell, the “hereditary signal” is involved into a system of dynamic self-organization of the organism. In essence, the functioning of all the mechanisms mentioned above (epigenetic regulation) should be considered in dynamics, that is, in the context of the entire morphoprocess 11, 37, 76. However, there is more to the regulative connections of the morphoprocess than epigenetic regulation. Cell communications and positional information are crucial for the morphoprocess of a multicellular organism, and so are systems determining the cell cycle length, the choice of cell fate, the character of growth and the ontogenetic features of the life cycle stages. On the whole, the implementation of a particular morphoprocess 37is characterized by a system of correlations, whose interactions can be considered as a dynamic self-organization with a high degree of equifinality.
The changes in the genome structure that fail to ensure the implementation of the ontogenesis would invariably result in the arrest of the morphoprocess and the death of the organism. Other changes involved in the dynamic self-organization of the morphoprocess are part and parcel of the stable functional system.
C. Filter no. 3. Physiological Regulation.
Physiological regulation is a system of mutual influences, including feedbacks, of the organism’s parts ensuring its functional integrity. An important feature of this level is the need to ensure effective performance under changing conditions of the environment. This involves: maintenance of the optimal level of metabolism depending on nutrition, breathing, osmoregulation and excretion; adequate intensity of locomotion; effective functioning of the integrating systems (circulatory system, neuroendocrine system, nervous system). The existence of an organism in a changing environment critically depends on dynamic correlations between these functions, that is, the system of compensations and feedbacks underlying the functional stability of the entire organism 109.
It is physiological regulation that, in combination with the dynamic impacts of the environment, brings out the implemented range of hereditary variation, referred to as phenotypic plasticity. From this viewpoint, the potential effectiveness of the physiological system filter is determined by the range of physiological tolerance and physiological resistance of an individual 13.
It is evident that the system of physiological regulation would compensate hereditary changes that may negatively affect some physiological parameters. At the same time, a considerable change in the morpho-functional characteristics of an individual would reflect the fact that the “hereditary signal” had passed through the first two system filters and failed to be compensated by the third. Then we may expect an individual to approach the limits of the resistance range. Its viability would decrease considerably and the probability of its death would increase dramatically. However, this would be the case only if the system filters of the higher levels fail, too (see below).
D. Filter no. 4. Behavioural Regulation.
The passage of the “hereditary signal” through epigenetic, ontogenetic and physiological “system filters” would herald the emergence of an individual whose functionality sets it aside from other individuals of the population. Then the filter of the next level comes into play.
Organisms interact with the environment through a system of their behavioural reactions. This system, formed in ontogenesis, is a powerful system filter on the way of the hereditary character to its reflection in the survival/fertility of the progeny of the organism. More or less considerable changes in the functional characteristics of an individual determined by its hereditary features would elicit corresponding behavioural modifications. From the point of view of an overall fitness of an individual, such modifications — behavioural programmes —would act as compensations improving survival and reproductive success. The resulting behavioural plasticity underlies the effectiveness of a vast range of morpho-functional variants present in the population of the species. Behavioural adaptations may prompt the choice of specific microhabitats and diet preferences, determine activity periods and the character of interactions with conspecific and heterospecifics. Formally, this system filter may be described as ensuring the existence of an organism and the implementation of its major functions in a heterogeneous environment.
The plasticity of behavioural mechanisms underlies the existence of a vast number of functionally different individuals in the population. Because of this plasticity, the diversity of morpho-functional variants in the population cannot be classified into a priori more or less successful (or unsuccessful), with the obvious exception of non-viable individuals. Indeed, the realization of these variants in a heterogeneous environment is mediated by behaviour. And, what is more, it is the diversity within the population that assures its stable existence as a system.
All the above “filters” represented systems of the self-organization of an organism (functioning, of course, in the context of environmental conditions). Yet though the systemic characteristics of an organism in the hierarchy of living systems (level of organization 10, 76, 88) are expressed most vividly, organisms themselves form part of more complex systems (complexity of organization 10). These supraorganismic systems — populations and communities — are distributive 111: the degree of their organization is lower than that of their constituting elements. However, they also have some features of systemic organization and so a certain degree of systemic integrity ensured by regulatory mechanisms. Earlier we have highlighted various aspects of systemic organization of an organism. Now it is time to look for systemic properties of more complex systems — populations and communities.
E. Filter no. 5. Populational Compensations
Should the “hereditary signal” pass through all the above compensatory filters, it would not necessarily mean that natural selection would immediately set to work. In this case, too, the “hereditary signal” cannot be directly translated into stable reproductive success (stable in the series of generations) or failure of the signal’s carriers. The potential relative importance of the “hereditary signal” for the reproduction of the population can only be determined in the context of the system of populational regulation. This system can be considered as the “filter” of the fifth level.
At least two groups of system mechanisms ensuring the integrity of the population as a system and its successful reproduction should be taken into account here. 1. A group of mechanisms of “populational mutual assistance” (see, e.g., 61). 2. A group of mechanisms ensuring populational compensations of abundance (birth and mortality rates, emigration and immigration). Moreover, it should be emphasized that populational compensations (feedbacks) may act during the lifetime of several generations. The impact of frequency-dependent differential mortality is one of such mechanisms 5,110.
So, the reproductive value of an individual — the carrier of the hereditary signal — may be identified only in the context of the surrounding conspecifics. Its hereditary morpho-functional characteristics (the signal that has passed through filters of levels 1—4) may, of course, correlate with its reproductive success. However, the strength of this correlation will be considerably modified by the system of populational interactions of the individual in question. The signal itself becomes distinctly conditional 11. That is, it can be assessed only in the context of interaction.
F. Filter no. 6. Biocenotic (ecosystem) Compensations.
So far, discussing populational compensations, we have considered only the organism’s interactions with conspecifics. This was justified as long as we had to identify the system of dynamic feedbacks at the population level. However, each individual is also involved in biocenotic interactions, and these should be taken into account as well. The passage of the “hereditary signal” through filters 1-4 would not only reserve its carrier a special place in the system of populational regulation (filter of the fifth level) but also, and simultaneously, alter the interactions of this individual with heterospecifics in the community. The change would manifest itself most conspicuously in the interactions of the individual with organisms whose populations form stable biocenotic links in the community (parasitic and predator-prey systems, mutualistic complexes of species etc.; 9). These diverse interactions are characterized by stable feedback-based regulations. A change in the properties of an element (individual with modified characters) would be necessarily expressed in compensatory changes of the interaction with the heterospecifics. From the point of view of the “hereditary signal” under discussion, this biocenotic complex of feedbacks is the filter of the sixth level. Within this filter, the correlation of the hereditary signal (which has passed through filters 1-4) with the reproductive success of an individual may level out. Or else the sign of the correlation may change from plus to minus or vice versa. Importantly, in this case as well as in the case of populational system filter the character is necessarily conditional. Its value may be assessed only in the context of the structure of a concrete biocenotic connex 9, moreover, in the context of a concrete phase of its dynamic state. A change in the properties of an individual becomes an indispensable structural-functional element in the system of regulatory links within the community. In a way, the “hereditary signal” becomes involved in the structure of the supraorganismic system. It is only from this viewpoint that its significance can be assessed.
Conclusion
Two major components should be considered when discussing whether the mechanism of natural selection does act in reality: the structure of variation and the complex of hierarchical “system filters”.
As concerns the structure of variation: we can conclude that new hereditary characteristics of individuals fall onto a narrow “strip of land” in the sea of potential possibilities (see Figure 1). Hereditary variation is non-homogenous, and very much so. This means that it is the characteristics of variation that determine the direction of potential evolutionary changes. It is utopian to think that natural selection has a virtually unlimited range of hereditary variations at its disposal. It does not, and this is due, first of all, to the character in which variation is manifested, its non-homogeneity. We have to admit that in the continuum of possible evolutionary mechanisms ranging from orthogenetic (a narrow range of the potentially possible variation is implemented) to selectogenetic (much of the potential variation is implemented) orthogenesis rules the day (see Figure 1).
Figure 1.Schematic representation of: А - frequency distributions of a character with a narrow (1,2) or broad (3,4) range of potential values; В – proportion of implemented variation (black sectors) in the potential range of variation of this character (circles under the abscissa). The proportion of implemented variation increases in the series 1 – 4. If the progeny of organisms is characterized by a small proportion of implemented variation (1, 2 В) and a narrow range of character implementation (1, 2 А), the evolutionary mechanisms of structural transformism (orthogenesis) prevail. If the progeny of organisms demonstrates a large proportion of potentially possible variants of a character (3, 4 В) and the values of the character vary broadly (3, 4 А), indirect adaptogenesis (selectogenesis) is possible. Accordingly, the role of natural selection increases from left to right along the abscissa.
Just how effective can selection be within the range of implemented variation, narrow as it is? Does dispersion of genotypes within this range correlate directly with differential mortality/contribution into the gene pool of the next generation?
Evidently, no. The consequences of changes in the genotype of an organism are involved into a system of hierarchical multiple compensation, from the molecular to the biocenotic level. For clarity, in this work we have represented this process as passage through a series of “system filters” (Figure 2). Each filter is represented by multiple feedbacks, compensatory reactions, which maintain the integrity of systems at the epigenetic, ontogenetic, physiological, behavioural, populational and biocenotic level. The first system filters (1-3) are responsible for the integrity of the organism as such. Filter 4 minimizes the costs of interaction of the organism with the environment in the broad sense of the word. Filters 5 and 6 characterize the integrity of supraorganismic systems. The importance of a given hereditary character for the reproduction success of its carrier cannot be assessed because the systems of self-organization at each level alter the value of the “hereditary signal”. Strictly speaking, hereditary change is a priori neutral, that is, neither positive nor negative. Characters of an organism are involved in the structure and functions of systems of a higher rank. The actual, “current” value of a given character makes sense only in dynamical interaction with the elements of these systems. Taken together, this means: any non-lethal character is conditional. The significance of a character for reproduction is dynamical and impossible to formalize.
Figure 2.Schematic representation of “system filters” at work. Successively increasing ellipses denote “system filters”: 1 – epigenetic; 2 – ontogenetic; 3 – physiological; 4 – behavioural; 5 – populational 6 – biocenotic. The black circle denotes a conditional change of the hereditary material of the organism. Arrows indicate successive passage of the signal through the system filters. As the signal passes through the filters, the adaptive significance of this hereditary change for the organisms in the structure of its population and community is formed.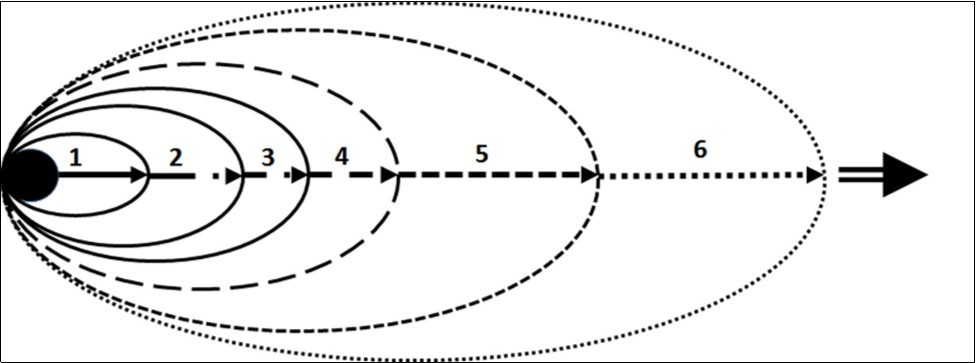
I would not want to convey a false impression that high mortality does not occur in natural populations and that all variation is involved in the self-organization of living systems. Clearly, this is not so. Numerous observations indicate that mortality may be high and variable. There are cases, especially among r-strategists, when much less than 1% of the progeny reaches the reproductive stage. The idea that I did want to convey was that mortality is not directly correlated with the character of variation. The “system filters” change any potential correlation beyond recognition, so that a direct correlation of the type “genotype — phenotype” becomes nonsensical. It does make sense, however, for one particular group of phenomena, which we will now discuss.
Of course, there are characters which, under given external conditions, exceed the limits of stability of the hierarchical systemic organization. These anomalous characters reflect hereditary changes whose consequences cannot be, as a rule, involved into systemic self-organization at any level. They lurk at the periphery of the implemented range of hereditary variants. Individuals with these characters die more often or fail to reproduce properly. The correlation between a given hereditary change and reproductive success (or lack thereof) is evident here. Natural selection may come into play. However, this exception highlights the general rule: the mechanism of natural selection has a small sphere of influence. It amounts to eliminating variants that cannot be involved into correlative systems of any level.
A dramatic change in environmental conditions may open a possibility for the anomalous characters to be involved in the general system of regulation. Viable individuals strikingly different from conspecific (“hopeful monsters”) may arise. The plausibility of such “systemic mutations” 33 and their potential evolutionary role are broadly discussed 39, 57, 116, 124.
Interestingly, such an approach also works a radical change on our understanding of the conditions most favourable for increasing the rate of evolution. An increased rate may be observed during periods when mortality is low, environmental conditions are favourable and natural selection is down to a minimum. On the contrary, a high mortality and the narrowing of the possible range of forms (that is, a high rate of natural selection) should slow down evolution dramatically. Hereditary changes entailing serious consequences, which cannot be involved into compensatory interactions, result in the death of individuals.
Taken together, the “system filters” described in this work form a unified and dynamic system of hierarchical multiple compensation. This system would “check” all hereditary changes of organisms (to note again, within the limited range of hereditary variation, see — variation). In other words, the consequences of hereditary changes are involved in a hierarchical system of multiple compensations. One may well say that this system and its functioning are self-organizing. Therefore, the adaptive nature of an individual is subject to the Law of Multilevel Self-Organization. It is here, in the crucible of “system filters”, that the adaptive nature of characters is formed. The variation of hereditary material is mere clay from which it is modelled.
A clear formulation of this law might make the case for the orthogenetic interpretation of evolution. The weakness of the orthogenetic approach is its failure to explain clearly the formation of adaptive characters underlying the fitness of an organism, in other words, purposefulness 77,78, see Lyubishchev 70 for the general discussion of the problem of purpose in evolution). At the same time, an increasingly critical attitude to selectogenetic explanations of the formation of purposefulness begs the question of the relative role of natural selection and self-organization in evolution 22, 35, 46, 66, 86, 87, 89. The mechanism suggested in this work implies that the hereditary signal itself (inherited change in the genotype) is not adaptive at all. Adaptive characters are formed in a hierarchical system of filters, from epigenetic to biocenotic.
In the light of the above, what can be said about the potential “creative” role of natural selection in the formation of adaptations and the change of the modal characters of species? We have to admit that the directionality of the evolutionary process is based on the limited range of hereditary variations. At the same time, the distribution of frequencies of different implemented mutations within this range is essentially non-homogenous. This consideration alone indicates that natural selection cannot determine the direction of the evolutionary process. During the development of an organism with certain hereditary changes the consequences of the “genetic signal” are involved in a multilevel system of correlations, into block reconstructions. If that happens, the correlation between the type of hereditary changes and the certainty of its contribution to the progeny becomes dynamic and conditional. It is so strongly disfigured that natural selection, stripped of its creative role, becomes entirely helpless.
Does natural selection still play a role in the functioning of living systems? Undoubtedly, it does. This is the role of a conservative mechanism eliminating the variants that, under given environmental conditions, cannot be integrated into the hierarchical system of filters. This mechanism prevents the implementation of the entire potential diversity of these systems. However, the significance of this phenomenon lies in a different plane. Therefore, it would be better, for the sake of clarity, to call it “differential mortality” rather than continue using the term “natural selection”, strongly linked with the idea of “creative evolutionary potential”.
Acknowledgments
I am eternally grateful Natalia Lentsman for her invaluable contribution to the English translation of the manuscript. This study was partially supported by a grant from the Russian Foundation for Basic Research (RFBR) №. 18-54-20001.
References
- 2.Alvarez-Buylla E R, Benítez M, Espinosa-Soto C. (2007) Phenotypic evolution is restrained by complex developmental processes. , HFSP J 1(2), 99-103.
- 3.Asis R A, Kondrashov E V, Koonin E V, Kondrashov F A. (2008) Nested genes and increasing organisational complexity of metazoan genomes. , TRENDS GENET 24, 475-478.
- 4.Ayala F J. (1982) Population and Evolutionary Genetics: A Primer.The Benjamin/Cummings Publishing Company,Inc.Menlo Park,London.
- 7.Beaber J W, Hochhut B, Waldor M K. (2004) SOS response promotes horizontal dissemination of antibiotic resistance genes. , Nature 427(6969), 72-74.
- 8.Beatty J. (2010) Reconsidering the importance of chance variation. In: Evolution. The extended synthesis. Eds M.Pigliucci,G.B.Muller.The MIT.Press,Cambrige. , Massachusetts, London, England 21-44.
- 10.Beklemishev V N. (1970) On general principles of organization of life. In: Biocenological basics of comparative parasitology. , Nauka, Moscow 7-25.
- 13.Berger. (1986) Adaptations of marine mollusks to the changes in environment salinity. , Leningrad
- 14.Berglund A, Rosenqvist G. (1986) Prawn Palaemon adspersus:. Effects on Growth and Predator Vulnerability. Oikos 46(3), 349-354.
- 15.Beverley S M, Coderre J A, Santi D V, Schimke R T. (1984) . Unstable DNA Amplifications in Methotrexate-Resistant Leishmania Consist of Extrachromosomal Circles which Relocalize during Stabilization. Cell 38, 431-439.
- 16.Bickford D, Lohman D L, Sodhi N S, PKL Ng, Meier R et al. (2006) Cryptic species as a window on diversity and conservation. , TRENDS ECOL EVOL 22(3), 148-155.
- 18.Brakefield P M. (2010) Radiations of Mycalesine Butterflies and Opening Up Their Exploration of Morphospace. , AM NAT 176: (77 – 87.
- 19.Browne R A. (1978) Growth, Mortality, Fecundity, Biomass and Productivity of Four Lake Populations of the Prosobranch Snail, Viviparus Georgianus. , Ecology 59, 742-750.
- 20.Collins J P. (1979) Intrapopulation Variation in the Body Size at Metamorphosis and Timing of Metamorphosis in the Bullfrog, Rana catesbeiana. , Ecology 604, 738-749.
- 21.Corey S, Reid D M. (1991) Comparative Fecundity of Decapod Crustaceans I. the Fecundity of Thirty-Three Species of Nine Families of Caridean Shrimp. , Crustaceana 60(3), 270-294.
- 22.Corning P A. (2002) The re-emergence of “emergence”: a venerable concept in search of a theory. , Complexity 7(6), 18-30.
- 23.Ch Darwin. (2009) On the Origin of Species by. Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. (6th ed.).Cambridge University Press,Cambrige .
- 24.Th Dobzhansky. (1970) . Genetics of the Evolutionary Process.Columbia , University Press,New York .
- 25.Danchin E, Charmantier A, Champagne F A, Mesoudi A, Pujol B et al. (2011) Beyond DNA: integrative inheritance into an extended theory of evolution. , Nature reviews, Genetics 12, 475-486.
- 27.CD Eds by Allis, Jenuwein T, Reinberg D, M-L Ass ed Caparros. (2007) . , New York, USA. Epigenetics
- 28.Fedoroff N, Schlappi M, Raina R. (1995) Epigenetic regulation of the maize Spm transposon. , BIOESSAYS 17, 291-297.
- 29.Fermin A C, RSJ Gapasin, Teruel M B. (2000) Spontaneous spawning, fecundity and spawning periodicity in the donkey’s ear abalone Haliotis asinina Linnaeus1758. In: Hylleberg A. (ed). Proceedings of the 10th International Congress and Workshop of the Tropical Marine Mollusc Programme. (TMMP),20-30 October1999,Hanoi and Haiphong, Vietnam. PhuketMarine Biological Center,Phuket.Phuket Marine Biological Center Special Publication 21(1), 195-201.
- 31.Galhardo R S, Hastings P J, Rosenberg S M. (2007) Mutation as a stress response and the regulation of evolvability. , CRIT REV BIOCHEM MOL 42(5), 399-435.
- 32.Gierl A. (1990) How maize transposable elements escape negative selection. , TRENDS GENET 6, 155-158.
- 34.Golubovskii M D. (2000) The age of genetics: evolution of ideas and concepts. Borei Art. , Saint Petersburg
- 35.Gould S J, Lewontin R C. (1979) The spandrels of San Marco and the Panglossian Paradigm: a critique of adaptacionist program. , P ROY SOC B-BIOL SCI 205, 581-598.
- 36.Granovitch A I, Yagunova E V, Maximovich A N, Sokolova I M. (2009) Elevated female fecundity as a possible compensatory mechanism in response to trematode infestation in populations of Littorina saxatilis. , (Olivi). INT J PARASITOL 39, 1011-1019.
- 37.Granovitch A I, Ostrovsky A N, Dobrovolsky A A. (2010) Morphoprocess and life cycles of organisms. , ZH OBSHCH BIOL 71(6), 514-522.
- 38.Granovitch A I, Iagunova E B, Sokolova I M. (2012) Trematode distribution in Littorina saxatilis populations can support the reproductive potential of the host: «toilers» and «idlers» among female periwinkles. , Parazitologiya 46(6), 444-462.
- 40.Haacke W.(1893) Gestaltung und Vererbung. Eine Entwickelungsmechanik der Organismen Weigel. , Leipzig
- 41.Hall B. (1990) Spontaneous point mutations that occur more often when advantageous than when neutral. , Genetics 126, 5-16.
- 42.Hall B. (1991) Adaptive evolution that requires multiple spontaneous mutations: mutations involving base substitutions. , P NATL ACAD SCI USA 88, 5882-5886.
- 43.Hastings P J, Rosenberg S M, Slack A. (2004) Antibiotic-induced lateral transfer of antibiotic resistance. , TRENDS MICROBIOL 12(9), 401-404.
- 44.Hausmann K, Hülsmann N, Radek R. (2003) . Protistology. E. Schweizerbart´sche Verlagsbuchhandlung. (Nägele u. Obermiller) , Stuttgart .
- 45.Havstad J C. (2011) Problems for natural selection as a mechanism. , Philosophy of science 512-523.
- 46.Hoelzer G A, Smith E, Pepper J W. (2006) On the logical relationship between natural selection and self-organization. , J EVOLUTION BIOL 19, 1785-1794.
- 48.Johannesson K, Panova M, Kemppainen P, André C, Rolán-Alvarez E et al. (2010) Repeated evolution of reproductive isolation in a marine snail: unveiling mechanisms of speciation. , Phil Trans R Soc B 365, 1735-1747.
- 49.Johnson B R, Lam S K. (2010) Self-jrganization, natural selection, and evolution: cellular hardware and genetic software. , BioOne 60(11), 879-885.
- 50.Karen G H, Hawley R S. (2007) Epigenetic regulation of chromosome inheritance. In: Allis CD, Jenuwein T, Reinberg D. (eds) Epigenetics.Cold Spring Harbour Laboratory Press,Cold Spring Harbour,New York,USA. 263-286.
- 52.Kim V N, Han J, Siomi M C. (2009) Biogenesis of small RNAs inanimals. , NAT REV MOL CELL BIO 10, 126-139.
- 53.Kirshner M W, Gerhart C G. (2010) Facilitated variation. In: Evolution. The extended synthesis. , Eds.M.Pigliucci, G.B.Muller.The MIT Press,Cambrige,Massachusetts,London,England 253-280.
- 54.Koonin E V. (2010) Taming of the Shrewd: novel eukaryotic genes from RNA viruses.doi:. , BMC Biol 8(2), 10-1186.
- 55.Koonin E V, Wolf Y I. (2009) Is evolution Darwinian or/and Lamarckian?. , BIOL DIRECT 4(42), 1-14.
- 56.Koonin E V. (2012) Logic of Chance. , The Nature and Origin of Biological Evolution.Pearson Education,Inc
- 57.Korochkin L I. (2002) Biology of individual development/ontogenesis. Moscow state university publishing. , Moscow
- 58.Kovalenko E E. (1996) Analysis of the Anura sacrum variability. 1. The method of analysis of the tailless amphibians sacrum variability. , ZOOL ZH 75(1), 52-66.
- 59.Kovalenko E E. (1996) Analysis of the Anura sacrum variability. Sacrum variability in the genus Rana. , ZOOL ZH 75(2), 222-236.
- 60.Kovalenko E E, Danilov I G. (2006) Variety of sacro-urostile sceleton in the family Bufonidae. (Amphibia, Anura).2.Analysis of the diversity by the method of spectra. , ZOOL ZH 85(6), 725-740.
- 62.Kull K. (2014) Adaptive evolution without natural selection. , Biological Journal of the Linnean Society 112, 287-294.
- 63.Kurakin A. (2008) Self-organization versus Watchmaker: stochastic dynamics of cellular organization. , Biological Chemistry 386, 247-254.
- 64.Laland K, Uller T, Feldman M, Sterelny K, G B Müller et al. (2014) Does evolutionary theory need a rethink?. , Nature 514(7521), 161.
- 66.Levy S F, Siegal M L. (2008) Network hubs buffer environment variation in Saccharomyces cerevisiae. , PLOS BIOL 6(11), 2588-2604.
- 67.Lewontin R C. Columbia University Press,New York. The Genetic Basis of Evolutionary Change (1974) .
- 68.Lissack M R. (1999) Complexity: The Science, its Vocabulary, and its Relation to Organizations. , Emergence 11, 110-125.
- 69.Loose M, Fischer-Friedrich M, Ries J, Kruse K, Schwille P. (2008) Spatial Regulators for Bacterial Cell Division Self-Organize into Surface Waves in Vitro. , Science 320, 789-792.
- 73.McClintock B. (1984) The significance of responses of the genome to challenge. , Science 26(4676), 792-801.
- 74.McElroy D W, Wuenschel M J, Press Y K, Towle E K, McBride R S. (2013) Differences in female individual reproductive potential among three stocks of winter flounder, Pseudopleuronectes americanus. , J SEA RES 75(1), 52-61.
- 75.McGhee G R. (2007) The Geometry of Evolution. Adaptive Landscapes and Theoretical Morphospaces.Cambridge.
- 76.McShea D W. (2001) The hierarchical structure of organisms: a scale and documentation of a trend in the maximum. , Paleobiology 27(2), 405-423.
- 77.Meyen S V.(2007a) The role of directed processes in evolution. In:. Ignatiev IA. (ed) SV Meyen paleobotanist, evolutionist, thinker. Geos , Moscow 244-287.
- 78.Meyen S V.(2007b) The morphology of Plants in the nomothetic aspect In:. Ignatiev IA. (ed) SV Meyen paleobotanist, evolutionist, thinker. Geos , Moscow 162-222.
- 79.Meyen S V.(2007c) Мейен СВ. About the ratio of nomogenetis and tichogenetic factors in evolution. In:. Ignatiev IA. (ed) SV Meyen paleobotanist, evolutionist, thinker. Geos , Moscow 229-243.
- 80.Meyen S V, Yu Chaikovsky. (1982) The works of A. Lyubishchev in general problems of biology. In:. , Problems of Form, Systematics and Evolution of Organisms. Nauka, Moscow, pp 5 – 23.
- 81.Millstein R L. (2013) Natural selection and causal productivity. H.-Chao et al.(eds.),Mechanism and causality. in biology and economics, history, philosophy and theory of life science.Springer Science+Business Media Dordrecht 147-163.
- 82.Misteli T. (2001) The concept of self-organization in cellular architecture. , The Journal of Cell Biology 155(2), 181-185.
- 83.Misteli T. (2007) Beyond the Sequence: Cellular Organization of Genome Function. , Cell 128, 787-800.
- 84.Motos L. (1996) Reproductive biology and fecundity of the Bay of Biscay anchovy population. (Engraulis encrasicolus L.). , SCI MAR 60(2), 195-207.
- 85.Newman S A. (2010) Dynamical patterning modules. In: Evolution. The extended synthesis. Eds.M.Pigliucci,G.B.Muller.The MIT Press,Cambrige,Massachusetts,London,England. 281-306.
- 86.Newman S A, Muller G B. (2010) Morphological evolution: epigenetic mechanisms. In: Encyclopedia of life sciences.(ELS).JohnWiley and Sons,Ltd:Chichester.
- 87.Nielsen R. (2009) Adaptacionism – 30 years after Gould and Lewontin. , Evolution 63(10), 2487-2490.
- 90.Osborn H F. (1929) The Titanotheres of ancient Wyoming, Dakota and Nebraska. United States Government Printing Office. , Washington
- 91.Paez-Espino D, Morovic W, Sun C L, Thomas B C, Ueda K et al. (2013) Strong bias in the bacterial CRISPR elements that confer immunity to phage. doi: 10.1038/ncomms2440. , Nat Commun 4, 1430.
- 92.Penteriani V, Otalora F, Ferrer M. (2006) Floater dynamics can explain positive patterns of density-dependent fecundity in animal populations. , AM NAT 168(5), 697-703.
- 93.Pigliucci M. (2010) Phenotypic plasticity. In: Evolution. The extended synthesis. , Eds.M.Pigliucci, G.B.Muller.The MIT Press,Cambrige,Massachusetts,London,England,pp 281-306.
- 94.Ponder R G, Fonville N C, Rosenberg S M. (2005) A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. , MOL CELL 19(6), 791-804.
- 95.Ponomarenko A G. (2005) Paleontological data on the origin of arthropods. In: Vorobyova EI, Striganov. (ed) Evolutionary factors of animal diversity formation.KMK Scientific Press,Moscow. 146-155.
- 96.Ponomarenko A G. (2008) Early stages of the arthropods’ evolution. In: Mamkaev YV. (ed) Evolutional morphology of animals. SPbSU publishing, Saint Petersburg. 43-57.
- 97.Skipper R A, Millstein R L. (2005) Thinking about evolutionary mechanisms: natural selection. Studies in History and Phylosophy of Biological and Biomedical Sciences 36, 327-347.
- 98.Quesada H, Posada В, Caballero A, Moran P, Rolan-Alvarez E. (2007) Phylogenetic evidence for multiple sympatric ecological diversification in a marine snail. , Evolution 61(7), 1600-1612.
- 99.Radman M. (1974) Phenomenology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis. In: Prakash L,Miller FSM,Lawrence C,Tabor HW. (ed)Molecular and Environmental aspects of Mutagenesis.Charles C. Thomas,Springfield, Ill. 128-142.
- 100.Raff R A, Kaufman T C. (1991) Embryos, genes, and evolution: the developmental-genetic basis of evolutionary change.Indiana.
- 101.Raup D. (1966) Geometric analysis of shell coiling: general problems. , Journal of Paleontology 40, 1178-1190.
- 102.Raup D. (1967) Geometric analysis of shell coiling: coiling in ammonoids. , Journal of Paleontology 41, 43-65.
- 104.Rio D C. (1990) Molecular mechanisms regulating Drosophila P-element transposition. , ANNU REV GENET 24, 5430-5478.
- 105.AYu Rozanov. (1973) Morphological evolution of Archeocyathids and questions of the definition of Lower Cambrian stages. , Nauka, Moskow
- 106.Rozhnov S V. (2005) Morphological patterns of formation and evolution of higher taxa echinoderms. In: Vorobieva E, Striganov M. (eds) Evolutionary factors of wildlife diversity.KMK Scientific Press,Мoskow. 156-170.
- 107.Rozhnov S V. (2006) The law of homologous series of N. Vavilov and archaic manifold according to paleontology. In: The evolution of the biosphere and biodiversity. y. KMK Scientific Press, Мoskow. 134-147.
- 108.Ruppert E E, Fox R S, Barnes R D. (2004) Invertebrate zoology. A functional evolutionary approach. (7-th Edition). Thomson Brooks/Cole. , Pacific Grove, CA
- 110.Schmalhausen. (1982) The organism as a whole in its individual and historical development. , Nauka, Moskow
- 113.Shapiro J A, Sternberg R. (2005) Why repetitive DNA is essential to genome function. , BIOL REW 80, 227-250.
- 114.Sharov A A, Igamberdiev A U. (2014) Inferring directions of evolution from patterns of variation: The legacy of Sergei Meien. , BioSystems 123, 67-73.
- 116.Stegnii V N. (1993) Architectonics of a genome, the system mutations and evolution.NovosibirskUniversityPress.
- 117.Tatarinov L P. (1976) Morphological evolution of Theriodonts and general questions of phylogenetics. , Nauka, Moskow
- 118.RDK Thomas, Reif W E. (1993) The skeleton space: a finite set of organic designs. , Evolution 47, 341-360.
- 119.Thompson D’Arci W. University Press,Cambrige (1992) On Growth and Form, abridged edition.Cambridgem.
- 120.Tronteli P, Fiser C. (2009) Cryptic species diversity should not be trivialized. , Systematics and Biodiversity 7(1), 1-3.
- 121.Ubukata T. (2005) Theoretical morphology of bivalve shell sculptures. , Palaeobiology 31, 643-655.
- 122.Vasil’yev A G, Vasil’yeva I A. (2009) Homological variability of morphological structures and epigenetic divergence among taxa: principles of population meronomy.KMK Scientific Press,Moskow.
- 125.Waddington C H. (1957) The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology.Allen and Unwin,London.
- 126.Wagner A. (2000) Robustness against mutations in genetic networks of yeast. , Nature Genetics 24, 355-361.
- 127.West-Eberhard M J. (2005) Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Science of the USA 102(suppl.1): 6543-6549.
- 128.Wray G A. (2010) Integrating genomics into evolutionary theiry. In: Evolution. The extended synthesis. Eds.M. Pigliucci, G.B. Muller.The , Cambrige, Massachusetts, London, England 97-116.
Cited by (3)
- 1.Granovitch A. I., 2021, , , 3(), 391, 10.1007/978-3-030-65536-5_13
- 2.Granovitch Andrei I., 2022, , , 5(), 223, 10.1007/978-3-031-04783-1_9
- 3.Miller William B., Baluška František, Reber Arthur S., Slijepčević Predrag, 2024, Biology in the 21st century: Natural selection is cognitive selection, Progress in Biophysics and Molecular Biology, 190(), 170, 10.1016/j.pbiomolbio.2024.05.001
