Triacontanol Alleviated Nickel Toxicity in Maize Seedling by Controlling Its Uptake and Enhancing Antioxidant System
Abstract
Triacontanol (TRIA) role in improving growth, physiological activities and tolerance against abiotic stresses has been reported. Yet, the mechanism by which TRIA executes its effects remains elusive. This work therefore studied the possible role of TRIA exogenous application in counteracting the adverse effects of nickel (Ni) treated maize seedlings. Maize seedlings (15-day-old) were grown in washed sand irrigated with nutrient solution provided with 100 μM NiCl2. Two concentrations of TRIA (25 and 50 µM) were applied twice as a foliar spray for Ni-stressed seedlings. Shoot and root growth attributes, Ni content, and antioxidant defence systems of maize seedlings were determined. Ni treatment reduced the shoot and root length and biomass, causing necrosis of the old leaves,greater reduction was shown in the roots. The shoot and root length was negatively correlated with their Ni content, which was consistent with their content of H2O2, but not with their malondialdehyde (MDA) content. As the roots had the greatest Ni content, maximum peroxidase (PX) and glutathione reductase (GR) activity as well as the highest ascorbic acid (ASA) and reduced glutathione (GSH) content were observed in the roots. The Ni-induced deleterious effects were alleviated by foliar application of TRIA concentrations. Also, TRIA treatment minimized root Ni content, whereas it maintained the shoots unharmed by Ni. Such mitigative effects of TRIA are explained by its key role in enhancing antioxidant capacity (expressed as IC50), increased PX and ascorbate oxidase (AO) activity, GSH, and total phenolic contents.
Author Contributions
Academic Editor: Mohamed Magdy Mansour, Department of Botany, Faculty of Science, Ain Shams University, Cairo 11566, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Abeer Abdelrazk Younis, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Heavy metal pollution is a global concern as it adversely affecting crop production. Heavy metals (HMs) are naturally occurring metals with atomic numbers greater than 20 and an elemental density greater than 5 g cm−31, 2. HMs including cadmium (Cd), lead (Pb), and mercury (Hg), are nonessential and highly toxic to plants 3, 4, 5. Other metals are required for life and considered as micronutrients (i.e., Zn, Mn, Ni, Cu, etc.), but their excessive accumulation in living organisms is always toxic. Ni is one of such micronutrients with dual characteristics. For instance, several enzyme activities depend on the presence of Ni highlighting its benefit effects on plant growth and development 6. Conversely, excess concentrations of Ni become toxic and cause disturbances in several physiological processes including photosynthesis, respiration, mineral nutrition, transport of assimilates and water relations 7. It is documented that the adequate levels of Ni for plant species are ranged from 0.01 to 10 mg g-1 dry weight 8.
Ni toxicity induces high levels of reactive oxygen species (ROS) which triggers lipid peroxidation, oxidation of proteins, degradation of chlorophyll pigments and DNA damage 9, 10, 11. Plants evolved a complex ROS scavenging mechanism at the molecular and cellular levels to survive with HMs stress 11. Therefore, increased stress tolerance in metal exposed plants is often associated with enhancement of antioxidant defense system comprising both enzymatic and non-enzymatic antioxidants 12, 13, 14. The antioxidant enzymes comprise superoxide dismutase (SOD), catalase (CAT), peroxidase (PX), polyphenol oxidase (PPO), glutathione reductase (GR), ascorbate peroxidase (APX), and ascorbate oxidase (AO) 14. The non-enzymatic antioxidants include phenolics, ascorbate (ASC), α-tocopherol, proline and glycinebetaine, and reduced glutathione (GSH) 15, 16, 17.
TRIA is one of relatively new plant growth regulators (PGRs) which has been established to play a critical role in plant growth and development when exogenously applied to various plant species 18, 19. The prominent effect of TRIA has been reported to influence the enzymes regulating growth 20, metabolic processes in plants 21, enhance photosynthetic rate and chlorophyll fluorescence 22, 23, stimulate mineral nutrients uptake 24, 25, and increase various organic compounds in plants 26. Furthermore, TRIA has been shown to improve the plant resistance against several abiotic stresses as salinity 23, 24, 14, water stress 27, chilling 28, and HMs stress 29, 30. To our knowledge, the role of TRIA in Ni-induced oxidative stress and antioxidant response is fragmentary studied. Maize (Zea mays L.) is the third most important cereals crop 31 cultivated globally and used largely as food for human and animals. Maize suffers heavily by metals which eventually reduce its growth and grain yield 32. The present study was therefore undertaken to investigate the role of TRIA in mitigating the adverse effects of Ni stress on maize seedlings. A variety of biochemical and physiological parameters related to antioxidant defense systems was addressed.
Materials and Methods
Plant Material and Growth Conditions
Maize (Zea mayes L.) grains (hybrid three way cross 321) were obtained from the Agricultural Research Centre, Giza, Egypt, and kept in the dark at 4 °C before use. The grains were surface sterilized by immersion in 1% (w/v) sodium hypochlorite solution for 30 min. Maize was cultivated in sand in plastic pots (diameter 15 cm, height 30 cm, 2.5 Kg dry sand per pot). The sand was washed with 12% hydrochloric acid to remove any carbonates and contaminants, rinsed with deionized water, and then dried in an oven (70 °C, 48 h, then 200 °C, 2 h). During the first week from the sowing, seedlings were irrigated with distilled water; then nutrients were added with Ni contamination (NiCl2.6 H2O), in a single dose (100 μM), in a 150 ml (about 0.25 mg Ni/ 100 g soil) nutritive solution was prepared according to the composition described by Smithet al. 33. The nutrient solutions were applied at a rate of 50 ml per pot three times a week to maintain the same quantity of nutrient solution per unit of sand. Moisture stress was avoided by watering the sand in the pots to 80 % of saturation capacity. Two concentrations of triacontanol solution (TRIA) (Triplntanol.com), at 25 and 50 µM, were applied as foliar spray treatment at the two-leaf stage (15-day-old seedlings) twice for 7 days. At the end of experiment, 21-day-old seedlings of both the treated and the untreated (control) shoot and root samples were collected, five plants per treatments were subjected for measuring some growth criteria and the remaining seedlings immediately frozen in liquid nitrogen and then stored at −80 °C for the analyses.
Chlorophyll Fluorescence Measurements
The maximum quantum efficiency of PSII(Fv/Fm) were determined on fully exposed leaves with a Hansatech Pocket Plant Efficiency Analyzer (Pocket PEA, Hansatech Instruments, King’s Lynn, Norfolk, UK) by following Kitajima and Butler 34 method. The data were recorded using previously dark-adapted leaves for 30 min.
Determination of Nickel Content
Dried samples (roots and shoots from each treatment were extracted by dry ashing as described by Chapman and Pratt 35. Ni content was determined by atomic absorption spectroscopy (Savant AA, GBC, Australia). The results were expressed as mg of metal g_1 of sample (dry weight).
Lipid Peroxidation and Hydrogen Peroxide Contents
DPPH Radical Scavenging Assay
The measurement of diphenylpicrylhydrazyl (DPPH) radical scavenging activity was carried out according to the method of Hatano et al. 38. The antiradical activity was finally expressed as IC50 (mg g-1 F W), the extract concentration required to cause a 50% inhibition. A lower IC50 value corresponds to a higher antioxidant activity of the plant extract. Standard curve was prepared to calculate IC50 value using ascorbic acid.
Enzymatic and Non-Enzymatic Antioxidants
Antioxidant enzymes were extracted from maize shoots and roots by using a known volume of phosphate buffer (PH 7) (1:4 W/V). The crude extracts were used for enzyme assays. Superoxide dismutase (SOD, EC 1.15.1.1) activity was assayed according to method for Kong et al. 39. Catalase (CAT, EC 1.11.1.6) activity assayed following the method of Aebi et al. 40. Peroxidase (PX, EC 1.11.1.7)) activity was determined by the method of Shannon et al. 41. Ascorbate oxidase (AO, EC 1.10.3.3) and peroxidase (APX, EC 1.11.1.11) activities were measured by the methods of Diallinas et al. 42 and Ali et al. 43 respectively. Glutathione Reductase (GR; EC1.6.4.2) was assayed by the method of Goldberg and Spooner 44 using a commercially kit (Biodiagnostics, Giza, Egypt). Polyphenol oxidase (PPO,EC 1.10.3.1) activity was measured by the method of Gonzalez et al. 45. Non-enzymatic antioxidants comprises total phenolics, as gallic acid equivalent (GAE), were determined according to Makkar et al. 46. Ascorbic acid (ASA) was determined by the methods of Mukherjee and Choudhuri 47. Reduced glutathione (GSH) was estimated according to the method given by Beutler et al. 48 using a commercially kit (Biodiagnostics, Giza, Egypt).
Statistical Analysis
The results were subjected to one-way analysis of variance (ANOVA) using the software package SPSS v20.0 (SPSS Inc., Chicago, USA). The comparison of the means of different treatments was carried out using Duncan’s multiple range test at a significance level of 5% (P ≤ 0.05).
Results
Maize seedlings exposed to Ni treatment exhibited a major decrease in shoot height, circumference and fresh weight, as well as root length (Figure 1A and Figure 2) and developed symptoms of Ni toxicity such as chlorosis and necrosis, especially in the older leaves (Figure 1B). However, a slight increment root fresh weight was observed (Figure 2C). Foliar-applied TRIA significantly improved Ni-induced reduction in growth traits, but, it did not have any noticeable effect on root fresh weight of maize seedlings (Figure 1 and Figure 2).
Figure 1. A) A Growth comparison of 21-day-old maize seedlings (control—untreated plants, Ni—plants exposed to Ni stress alone, Ni+ TRIA 25—plants exposed to Ni stress and treated with 25 μM triacontanol, Ni+ TRIA 50—plants exposed to Ni stress and treated with 50 μM triacontanol). B) Symptoms of injury on leaf tips, especially in mature leaves, caused by Ni exposure.
Figure 2. Effect of Nickel (Ni) alone or combined with different concentrations of triacontanol (25 and 50 μM) on A) Shoot and root length, B) Shoot circumference and C) Shoot and root fresh weights, as compared with the control untreated plants. Data are means ± SD (n=4), bars with different letters are significantly different at P ≤ 0.05.
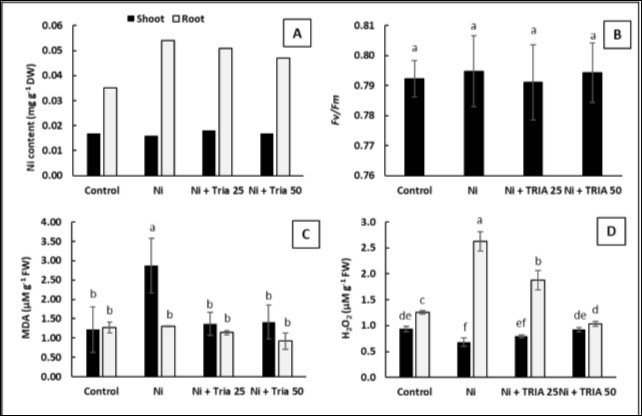
Application of TRIA (50 μM) markedly decreased root Ni content, however, both TRIA treatments (25 and 50 μM) did not show any prominent effect on shoot Ni content (Figure 3A). Meanwhile, Ni effect was emphasized by correlating Ni content with root growth of maize seedling, as it has revealed a strong reverse correlation with root length (R2= 0.9, data not shown), while a positive correlation has been obtained with root fresh weight (R2= 0.9, data not shown).
The values of the maximum quantum efficiency of PSII(Fv/Fm) showed nonsignificant effect for Ni treatment as well as both concentration of TRIA (Figure 3B), which correlated with shoot Ni content (R2= 0.8, data not shown). Interestingly, Ni-exposed maize shoots exhibited higher lipid peroxidation (MDA) content reached about 136% as compared with untreated control seedlings despite of low shoot Ni content (Figure 3C). On the other hand, roots did not exhibit differences in MDA levels of Ni-exposed plants as well as TRIA-treated ones (Figure 3C). However, TRIA treatments decreased the MDA level by about its half value as compared with untreated Ni-stressed shoot (Figure 3C). Further, the exposure of maize seedlings to 100 μM Ni led to a significant increment of H2O2 content in the roots reached about 109% as compared with untreated control seedlings (Figure 2D). Meanwhile, applications of 25 and 50 μM TRIA significantly reduced H2O2 accumulated in Ni-stressed root by about 28.5% and 60.5% respectively (Figure 3D). The enhancement in root H2O2 level was positively correlated with its Ni content (R2= 0.7, data not shown). While in shoots, H2O2 were shown to be reduced in Ni exposed plants, but the treatment with TRIA resulted in H2O2 content to around control levels (Figure 3D).
Figure 3. Effect of triacontanol (TRIA) (25 and 50 μM) on A) Nickel (Ni) content B) Quantum efficiency of the photochemical reactions in PSII (Fv/Fm) in leaves, C) lipid peroxidation (MDA) and D) H202 content in shoots and roots of maize plants exposed to Ni stress, as compared with the control untreated plants. Data are means ± SD (n=3), bars with different letters are significantly different at P ≤ 0.05.
The lowest IC50 values indicating the highest antioxidant capacity was recorded in 25 μM TRIA-treated stressed shoots, whereas, the highest IC50 value was obtained in Ni-stressed roots of maize seedlings (Figure 4A). Which was strongly correlated with Ni content (R2= 0.91, data not shown). Regarding antioxidant enzymes, roots of all treatment showed boosted activities of most enzymes (SOD, CAT, PX, APX, GR and PPO), as compared with its corresponding roots (Figure 4). Reduction of SOD was resulted in 25 μM TRIA treated shoot, while no significant change in this enzyme activity for all root treatment (Figure 4B). Generally, maize roots exhibited increased CAT activities compared to those of roots (ranging between 1 and1.5 folds, except for 50 μM TRIA). The treatment does not seem to affect the CAT activity in both shoots and roots. The only exception is the treatment with 50 μM TRIA, in which the treatment caused a reduction in root’s CAT activity to be almost equal to that of shoot (Figure 4C). Ni-stressed maize roots recorded the maximum PX activity (115.4% over the control level), while no significant changes of PX activity was recorded in its corresponding shoots (Figure 2D). Further, the TRIA application reduced the activity of PX enzyme in both Ni-stressed shoots and roots, with greatest decrease in 25 μM TRIA-treated shoots (ten folds blow the untreated control) (Figure 4D). On the other hand, significant reductions in APX enzyme activity by 25% and 22% were noticed in 50 μM TRIA-treated shoots and roots respectively as compared with Ni-stressed untreated ones (Figure 2E). The most pronounced induction in response of maize plants to Ni stress was observed in the case of GR activity of the maize roots, where it recorded about 9-fold higher than the control (Figure 4F). However, no significant changes in GR activity were found in the maize Ni-stressed shoots as well as TRIA treatment (Figure 4F). Shoots treated with 25 μM TRIA exhibited the maximum enhancement in AO activity under Ni stress (Figure 4G). While in roots, 25 μM TRIA treatment significantly reduced the activity of this enzyme by about 61% as compared with the untreated stressed roots (Figure 4G). Treatment with 100 μM Ni along with 25 μM TRIA reduced the PPO activity by 43.7% (Figure 4H).
Figure 4. Effect of triacontanol (TRIA) (25 and 50 μM) on A) scavenging activity of DPPH expressed as IC50 value B) Superoxide dismutase (SOD) activity, C) Catalase activity (CAT), D) Peroxidase activity (PO), E) Ascorbate peroxidase (APX) activity, F) Glutathione reductase (GR), G) Ascorbate oxidase (AO) activity and H) Polyphenol oxidase (PPO) activity measured in shoots and roots of maize plants exposed to Ni stress, as compared with the control untreated plants. Data are means ± SD (n=3), bars with different letters are significantly different at P ≤ 0.05.
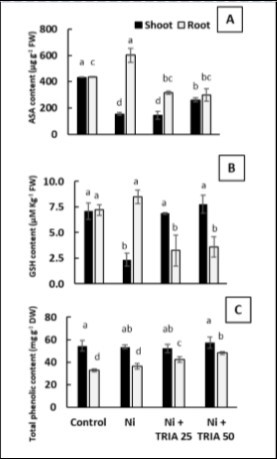
Interestingly maize roots revealed similar behavior in both ASA and GSH contents (Figure 5A and B). As Ni caused a significant increase in ASC and GSH contents in roots of maize seedlings reached about 38.5% and 18% respectively as compared with unstressed control (Figure 5A and B). However, both TRIA treatments (25 and 50 μM) resulted in significant reduction in ASC and GSH contents of Ni-stressed maize roots (Figure 5A and B). On the other hand, in the shoots, treatment with Ni induced a highly significant reduction approximately 64% and 67% in ASC and GSH contents respectively as compared with untreated control (Figure 5A and B). Meanwhile, both TRIA treatment succeeded to retrieve control levels of GSH, but for ASA in maize shoots (Figure 5A and B). Interestingly, total phenolic compounds were the unique antioxidant component that accumulated in shoots more than roots, even though their contents seemed to be treatment independent (Figure 5C). Total phenolic of roots has not been affected by Ni stress, but it is boosted by addition of TRIA in concentration dependent manner (Figure 5C).
Figure 5. Effect of triacontanol (TRIA) (25 and 50 μM) on A) Ascorbic acid content (ASA) B) Reduced glutathione content and C) Total phenolic content of maize plants exposed to Ni stress, as compared with the control untreated plants. Data are means ± SD (n=3), bars with different letters are significantly different at P ≤ 0.05.
Discussion
Decreased growth of maize seedlings exposed to 100 µM Ni could be attributed to inhibition of cell division 7, 49. Several studies indicated that Ni-stressed plants showed disrupted mitotic index and had chromosome abnormalities 50, 51, 7, 52, 53. TRIA-induced improvement in the growth of maize might be due to the synergistic interaction of TRIA with phytohormones and induction of 9-b-L (+) adenosine, cytokinin like structure, which might mainly responsible for increased growth 26. Our results are in agreement with previous studies where TRIA induces the growth of Brassica napus53, Coriandrum sativum30, and Erythrina variegata29 under metal stress.
Increased Ni accumulation in the roots but not in the shoots of maize seedlings could possibly be due to the contention that roots are directly contacted with soil solution including Ni, and root water absorption likely increased Ni accumulation with low translocation of Ni to the shoots 54. Consistently, previous works on other plant species also reported a greater accumulation of Ni in the roots 55, 56, 57, 58. TRIA treatment decreased root Ni content in concentration dependent manner, but never retrieved the control levels. Accordingly, it seems that TRIA might reduce Ni uptake from the beginning. Supporting to our proposal is the finding that TRIA could affect heavy metal ATPases or cation diffusion facilitators 1. Although little is known about the effect of TRIA on ions absorption and membrane permeability, Ramani and Kannan 58 showed that TRIA hinders absorption of minerals from the soil. Moreover, Shripathi and Swamy 59 report changes in the composition of membrane phospholipids of cotton by TRIA treatment.
Our results showed nonsignificant effect on Fv/Fm values in all treatments of maize shoots, which is consistent with low Ni content in the shoots. Absence of Ni effect on this parameter might be interpreted to short time of Ni exposure independently of the prior spraying of the seedlings with TRIA. Also, lower contents of H2O2 in the shoots of Ni stressed seedlings parallel with shoot decreased Ni concentration. In contrast, significant increase in the shoot MDA relative to the roots under Ni stress might be explained by the fact that other ROS might participate in increased MDA and that symptoms of Ni toxicity such as chlorosis and necrotic spots were visible in the older leaves of maize plants treated with Ni which may indicate Ni accumulation mostly in the oldest leaves and hence function as metal sink and protect younger leaves against its toxicity 60. The results agree with earlier studies on Ni-stressed shoots of Cajanuscajan, Brassica juncea61, 62, and Zea mays 52. Similarly, roots of Triticum aestivum54Solanum nigrum51 did not demonstrate significant changes in lipid peroxidation levels under Ni stress. The reduction in the shoot lipid peroxidation by foliar treatment of TRIA under Ni stress is in agreement with previous investigations 30, 14 that TRIA may play a key role in protecting the structure and function of cell membranes against metal toxicity via elevating antioxidant system. Maize shoots showed higher antioxidant capacities (expressed as lower IC50 of DPPH), which might be attributed to non-enzymatic antioxidant compounds like total phenolic compounds and reduced glutathione that accumulated in shoots in response to TRIA treatment.
Accumulation of H2O2 and Niin the roots of maize seedlings agrees with previous studies on roots of Alyssum bertolonii and Nicotiana tabacum63 and Triticum aestivum55, 56 illustrating Ni-stressed roots suffered from oxidative stress. Despite the higher Ni and H2O2 accumulation in roots, no significant effect on the root MDA which may be attributed to the elevated activity of antioxidant enzymes that scavenged ROS generated under stress conditions 55, 64, 65. Minimum antioxidant capacity was observed in Ni-stressed maize roots under TRIA treatment. Similar relation between Ni concentration and antioxidant capacity has been reported by Stanisavljevic et al. 66, Kulbat and Leszczyńska 67 and Georgiadou et al. 68. Interestingly, Ni content was found to be strongly correlated to IC50 and PX (R² = 0.91 and 0.93, respectively) and to lesser extent to APX, total phenolic compounds and H2O2 content (R² = 0.56, 0.55 and 0.68, respectively). This can be interpreted that accumulated Ni in stressed tissues exerted an oxidative stress leaded to H2O2 formation, which was scavenged using antioxidative enzymes and phenolic compounds. Similarly, Ibrahim et al. 69 report such correlation relationships under heavy metal stress. Moreover, our results observed a correlation between PX and both IC50 and H2O2 content, indicating that it is the chief antioxidant enzyme in Ni-stressed roots (either treated with TRIA or no). In addition to PX, Ni-stressed root used other antioxidative mechanisms, such as glutathione/ascorbate cycle, which was evident from their enhanced levels of GR, GSH and ASA. Despite these defense mechanisms, it appears that the oxidative rate was greater than scavenging one in maize stressed roots.
TRIA enhanced enzymatic (mainly peroxidases) and non-enzymatic (phenolic compounds) antioxidants under Ni stress, which is previously reported in response to heavy metal-stressed plants 30, 14, 54. Even though TRIA-treated plants did not use multiple and complex antioxidant systems as that observed in Ni-stressed plants alone, it seems that such antioxidant defense system was sufficient to reduce H2O2 content and counterbalanced the deleterious impacts of Ni stress. The elevated AO activity in of TRIA 25 μM treated shoots was one of the most remarkable results in this study as this was escorted by increased growth parameters and reduced ASA. Taken together, we can hypothesize that TRIA might have enhanced shoot growth via enhancing AO activity on the expense of ASA. AO is an ubiquitous apoplastic multi-copper oxidase enzyme that catalyze oxidation of apoplastic ASA into monodehydroascorbate (MDHA) then finally into dehydroascorbate (DHA) using oxygen as a hydrogen acceptor. This mechanism controlled by AO is the main regulator of apoplastic redox status and hence growth. The reciprocal interaction between AO expression and auxins has confirmed the role of AO in auxins signal transduction 70, 71. Smirnoff 72 has demonstrated that increased AO activity and its product (DHA) are performing critical role in auxin signal transduction and are directly associated with cell elongation and expansion in actively growing tissues. It has been also shown that overexpression of AO gene is associated with a leap in AO activity and apoplastic DHA, which promote shoot elongation in tobacco and cotton plants73, 74.
In conclusion, our results showed that TRIA foliar spray did not only alleviate the toxicity and oxidative stress imposed by Ni stress, but also provoked the growth to be comparable with that of the untreated plants. We propose three mechanisms by which TRIA express its effect: extrusion of Ni from roots, enhancing enzymatic and non-enzymatic antioxidants, and stimulating growth-related enzymes.
Acknowledgements
The authors would like to thank Dr Fatma Zaki for providing triacontanol. We specially thank Miss Eman Kamal for her help in the statistical analysis.
Abbreviations
References
- 1.Singh S, Parihar P, Singh R, V P Singh, S M Prasad. (2016) Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. , Front. Plant Sci 6, 1143.
- 2.Ali H, Khan E. (2018) What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition”:. , Toxicol. Environ. Chem. in Taylor and Francis 100(1), 6-19.
- 3.Ding S, Ma C, Shi W, Liu W, Lu Y. (2017) Exogenous glutathione enhances cadmium accumulation and alleviates its toxicity in Populus × canescens. , Tree Physiol 37(12), 1697-1712.
- 4.Ma C, Chen Y, Ding S, Li Z, Shi W. (2018) Sulfur nutrition stimulates lead accumulation and alleviates its toxicity in Populus deltoides. , Tree Physiol 38(11), 1724-1741.
- 5.Shi W G, Liu W, Yu W, Zhang Y, Ding S. (2019) Abscisic acid enhances lead translocation from the roots to the leaves and alleviates its toxicity in Populus. , J. Hazard. Mater.362 275-285.
- 6.Muszyńska E, Labudda M. (2019) Dual role of metallic trace elements in stress biology—from negative to beneficial impact on plants. , Int. J. Mol. Sci 20(13), 3117.
- 7.Muhammad B H, Shafaqat A, Aqeel A, Saadia H.Muhammad AFet al.(2013) Morphological, physiological and biochemical responses of plants to nickel stress: A review. , African J. Agric 8(17), 1596-1602.
- 8.Gratão P L, G B Pompeu, F R Capaldi, V A, Lea P.J,et al.(2008) Antioxidant response ofNicotiana tabacumcv. , Bright Yellow 2, 73-83.
- 9.Ahmad P, Jaleel C A, Salem M A, Nabi G, Sharma S. (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. , Crit. Rev. Biotechnol 30(3), 161-175.
- 10.M N Dourado, M R Franco, L P Peters, P F Martins, Souza L A. (2015) Antioxidant enzymes activities ofBurkholderiaspp. strains—oxidative responses to Ni toxicity. , Environ. Sci 22(24), 19922-19932.
- 11.Hasanuzzaman M, Nahar K, M, Roychowdhury R, Fujita M. (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. , Int. J. Mol. Sci 14(5), 9643-9684.
- 12.Fidalgo F, Freitas R, Ferreira R, A M Pessoa, Teixeira J. (2011) nigrumL. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. , Environ. Exp. Bot 72(2), 312-319.
- 13.Fidalgo F, Azenha M, A F Silva, A de Sousa, Santiago A. (2013) Copper-induced stress inSolanum nigrumL. and antioxidant defense system responses. Food Energy Secur. 2(1), 70-80.
- 14.Asadi Karam E, Keramat B, Sorbob S, Maresca V, Asrar Z. (2017) Interaction of triacontanol and arsenic on the ascorbate-glutathione cycle and their effects on the ultrastructure inCoriandrum sativumL. , Environ. Exp. Bot 14, 161-169.
- 15.Sinha S, Saxena R. (2006) Effect of iron on lipid peroxidation, and enzymatic and non-enzymatic antioxidants and bacoside-A content in medicinal plantBacopamonnieriL. , Chemosphere 62(8), 1340-50.
- 16.Ali EF MansourMMF. (2017) Glycinebetaine in saline conditions: an assessment of the current state of knowledge. , Acta Physiol. Plant 39, 56.
- 17.MMF Mansour, Ali E F. (2017) Evaluation of proline functions in saline conditions. , Phytochemistry 140, 52-68.
- 18.Perveen S, Shahbaz M, Ashraf M. (2013) Influence of foliar-applied triacontanol on growth, gas exchange characteristics, and chlorophyll fluorescence at different growth stages in wheat under saline conditions. , Photosynthetica 51(4), 541-551.
- 19.Verma A, C P Malik, V K Gupta, B K. (2011) Effects of in vitro triacontanol on growth, antioxidant enzymes, and photosynthetic characteristics inArachishypogaeaL. , Brazilian J. Plant Physiol 23(4), 271-277.
- 20.Chen X, Yuan H, Chen R, Zhu L, Du B. (2002) Isolation and characterization of triacontanol-regulated genes in rice (Oryza sativaL.): Possible role of triacontanol as a plant growth stimulator. , Plant Cell Physiol 43(8), 869-876.
- 21.D J Morré, Selldén G, X Z Zhu, Brightman A. (1991) Triacontanol stimulates NADH oxidase of soybean hypocotyl plasma membrane. , Plant Sci 79(1), 31-36.
- 22.Perveen S, Shahbaz M, Ashraf M. (2010) Regulation in gas exchange and quantum yield of photosystem II (PSII) in salt-stressed and non-stressed wheat plants raised from seed treated with triacontanol. , Pak. J. Bot 42, 3073-3081.
- 23.Shahbaz M, Noreen N, Perveen S. (2013) Triacontanol modulates photosynthesis and osmoprotectants in canola (BrassicanapusL.) under saline stress. , J. Plant 8(4), 350-359.
- 24.Perveen S, Shahbaz M, Ashraf M. (2012) Changes in mineral composition, uptake and use efficiency of salt stressed wheat (Triticumaestivuml.) plants raised from seed treated with triacontanol. , Pak. J. Bot 44, 27-35.
- 25.Chen X, Yuan H, Chen R, Zhu L, He G. (2003) Biochemical and photochemical changes in response to triacontanol in rice (Oryza salivaL.). , Plant Growth Regul 40(3), 249-256.
- 26.Naeem M, Khan A, Masroor M, Moinuddin. (2012) Triacontanol: A potent plant growth regulator in agriculture. , Journal of Plant Interactions 7(2), 129-142.
- 27.Muthuchelian K, Murugan C, Nedunchezhian N, Kulandaivelu G. (1997) Photosynthesis and growth ofErythrinavariegataas affected by water stress and triacontanol. , Photosynthetica 33(2), 241-248.
- 28.Z K Blamowski. (2009) The effects of triacontanol ‘TRIA’ and Asahi SL on the development and metabolic activity of sweet basil (OcimumbasilicumL.) plants treated with chilling. , Folia Horticulturae 21, 39-48.
- 29.Muthuchelian K, Bertamini M, Nedunchezhian N. (2001) Triacontanol can protectErythrinavariegatafrom cadmium toxicity. , J. Plant Physiol 158(11), 1487-1490.
- 30.Asadi Karam E, Keramat B, Asrar Z, Mozafari H. (2016) Triacontanol-induced changes in growth, oxidative defense system in Coriander (Coriandrum sativum) under arsenic toxicity. , Indian J. Plant Physiol 21(2), 137-142.
- 31.U B Ewa, Prince A, Jude O, Martins A. (2012) Evaluation of Agronomic Performance of Maize (Zeamays L.) under Different Rates of Poultry Manure Application in an Ultisol of Obubra, Cross River State. , Nigeria. Int. J. Agric. For 2(4), 138-144.
- 32.Aliu S, Gashi B, Rusinovci I, Fetahu S, Vataj R. (2013) Effects of some heavy metals in some morpho-physiological parameters in maize seedlings. , Am. J. Biochem. Biotechnol 9(1), 27-33.
- 33.G S Smith, C M Johnston, I S Cornforth. (1983) Comparison of nutrient solutions for growth of plants in sand culture. 94(4), 537-548.
- 34.Kitajima M, W L Butler. (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. , BBA - 376(1), 105-115.
- 35.Chapman H D, Pratt P F. (1962) Methods of analysis for soils, plants and waters. , Soil Science 93(1), 68.
- 36.Heath R L, Packer L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. , Arch. Biochem. Biophys 125(1), 189-198.
- 37.Velikova V, Yordanov I, Edreva A. (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. , Plant 151(1), 59-66.
- 38.Hatano T, Kagawa H, Yasuhara T, Okuda T. (1988) Two New Flavonoids and Other Constituents in Licorice Root Their Relative Astringency and Radical Scavenging Effects. , Chem. Pharm 36(6), 2090-2097.
- 39.F X Kong, Hu W, S Y Chao, W L Sang, L S Wang. (1999) Physiological responses of the lichenXanthoparmeliamexicanato oxidative stress of SO2. , Environ. Exp 42(3), 201-209.
- 41.L X Shannon, Kay E, J Y Lew. (1966) Peroxidase Isozymes from Horseradish Roots I. Isolation and physical properties.
- 42.Diallinas G, Pateraki I, Sanmartin M, Scossa A, Stilianou E. (1997) Melon ascorbate oxidase: Cloning of a multigene family, induction during fruit development and repression by wounding. , Plant Mol 34(5), 759-770.
- 43.Ali M B, Hahn E J, Paek K Y. (2005) Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. , Environ. Exp. Bot 54(2), 109-120.
- 44.D M Goldgerg, R J Spooner. (1983) Glutathion reductase, inMethods of Enzymatic Analysis ,3rd,ed. 258-265.
- 45.Trejo-Gonzalez A, Soto-Valdez H. (1991) Partial Characterization of Polyphenoloxidase Extracted from ‘Anna’. , Apple. J. Amer. Socie. for Horti. Sci 116(4), 672-675.
- 46.Makkar H P, Blümmel M, Borowy N K, Becker K. (1993) Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. , J. Sci. Food Agric 61(2), 161-165.
- 47.Mukherjee S P, Choudhuri M A. (1983) Implications of water stress‐induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. , Physiol. Plant 58(2), 166-170.
- 48.Beutler E, Durgun O, Kelly B M. (1963) Improved method for the determination of blood glutathione. , J. Lab. Clin. Med 61, 882-888.
- 49.Yusuf M, Fariduddin Q, Hayat S, Ahmad A. (2011) Nickel: An overview of uptake, essentiality and toxicity in plants. , Bull. Environ. Contam. Toxicol 86(1), 1-17.
- 50.Gomes-Junior R A, Moldes C A, Delite F S, Gratão P L.Mazzafera Pet al.(2006) “Nickel elicits a fast antioxidant response in Coffea arabica cells. , Plant Physiol. Biochem 44(56), 420-429.
- 51.Soares C, A de Sousa, Pinto A, Azenha M.Teixeira JVet al.(2016) Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake inSolanum nigrumL. under Ni stress. , Environ. Exp. Bot 122, 115-125.
- 52.Rizvi A, Khan M S. (2016) Heavy metal-mediated toxicity to maize: oxidative damage, antioxidant defence response and metal distribution in plant organs. , Int. J. Environ. Sci. Technol 16(8), 4873-4886.
- 53.Asadi Karam E, Maresca V, Sorbo S, Keramat B, Basile A. (2017) Effects of triacontanol on ascorbate-glutathione cycle inBrassicanapusL. exposed to cadmium-induced oxidative stress. , Ecotoxicol. Environ. Saf 144, 268-274.
- 54.Gajewska E, Słaba M, Andrzejewska R, Skłodowska M. (2006) Nickel-induced inhibition of wheat root growth is related to H2O2production, but not to lipid peroxidation. , Plant Growth Regul 49(1), 95-103.
- 55.Gajewska E, Skłodowska M. (2008) Differential biochemical responses of wheat shoots and roots to nickel stress: Antioxidative reactions and proline accumulation. , Plant Growth Regul 54(2), 179-188.
- 56.Singh K, Pandey S N. (2011) Effect of nickel-stresses on uptake, pigments and antioxidative responses of water lettuce,PistiastratiotesL. , J. Environ. Biol 32(3), 391-394.
- 57.González C I, Maine M A, Cazenave J, Hadad H R, Benavides M P. (2015) Ni accumulation and its effects on physiological and biochemical parameters ofEichhorniacrassipes. Environ.Exp Bot,117:.
- 58.Ramani S, Kannan S. (1980) Effect of triacontanol on the absorption and transport of Rb+and PO4−in plants. Zeitschrift für Pflanzenphysiologie. 99(5), 427-433.
- 59.Shripathi V, Swamy G S. (1994) Effect of triacontanol on the lipid composition of cotton (GossypiumhirsutumL.) leaves and its interaction with indole-3-acetic acid and benzyladenine. , Plant Growth Regul 14(1), 45-50.
- 60.Gajewska E, Skłodowska M. (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. , BioMetals 20(1), 27-36.
- 61.Madhava Rao KV, TVS Sresty. (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanuscajan(L.) Millspaugh) in response to Zn and Ni stresses. , Plant Sci 157(1), 113-128.
- 62.Ali B, Hayat S, Fariduddin Q, Ahmad A. (2008) 24-Epibrassinolide protects against the stress generated by salinity and nickel inBrassicajuncea. , Chemosphere 72(9), 1387-1392.
- 63.Boominathan R, Doran P M. (2002) Ni-induced oxidative stress in roots of the Ni hyperaccumulator,Alyssumbertolonii. , New Phytol 156(2), 205-215.
- 64.Gill S S, Tuteja N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 48(12), 909-930.
- 65.Hayat S, Hayat Q, Alyemeni M N, Wani A S.Pichtel Jet al.(2012) Role of proline under changing environments: A review. Plant Signaling and Behavior. 7(11), 1456-1466.
- 66.Stanisavljević N, Savić J, Jovanović Ž, Miljuš-Djukić J.Radović Set al.(2012) Antioxidative-related enzyme activity in Alyssum markgrafii shoot cultures as affected by nickel level. , Acta Physiol. Plant 34(5), 1997-2006.
- 67.Kulbat K, Leszczyńska J. (2015) Biotechnology and Food Science Antioxidants as a defensive shield in thyme (Thymus vulgarisL.) grown on the soil contaminated with heavy metals.
- 68.Georgiadou E C, Kowalska E, Patla K, Kulbat K.Smolińska B,et al.(2018) Iluence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, protnfeome regulation and allergen production in basil (OcimumbasilicumL.) plants. , Front. Plant Sci 9, 826.
- 69.Ibrahim M, Chee Kong Y, Mohd Zain N. (2017) . Effect of Cadmium and Copper Exposure on Growth, Secondary Metabolites and Antioxidant Activity in the Medicinal Plant Sambung Nyawa (Gynuraprocumbens(Lour.) Merr). Molecules 22(10), 1623.
- 70.Pignocchi C, Kiddle G, Hernández I, Foster S J.Asensi Aet al.(2006) Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. , Plant Physiol 141(2), 423-435.
- 71.Stevens R, Truffault V, Baldet P, Gautier H. (2018) Ascorbate oxidase in plant growth, Development, and stress tolerance. in Ascorbic Acid in Plant Growth, Development and Stress Tolerance 273-295.
- 72.Smirnoff N. (1996) The function and metabolism of ascorbic acid in plants. , Ann. Bot 78(6), 661-669.
Cited by (20)
This article has been cited by 20 scholarly works according to:
Citing Articles:
Journal of Crop Health (2025) Crossref
Hossam S. El-Beltagi, Mohamed Gad, Mohamed Abdel-Haleem, Tarek A. Shalaby, Adel A. Rezk et al. - Journal of Crop Health (2025) Semantic Scholar
Deleted Journal (2025) OpenAlex
Toxics (2024) Crossref
Saba Mudassar, Shakil Ahmed, R. Sardar, Nasim Ahmad Yasin, Muhammad Jabbar et al. - Toxics (2024) Semantic Scholar
Toxics (2024) OpenAlex
S. Tabur, Ş. B. Yilmaz Ergün, Serkan Özmen - Caryologia (Firenze) (2024) Semantic Scholar
Caryologia (2024) OpenAlex
Plants (2023) Crossref
Shakil Ahmed, Minahil Amjad, R. Sardar, M. H. Siddiqui, M. Irfan - Plants (2023) Semantic Scholar
Plants (2023) OpenAlex
Chemosphere (2022) Crossref
Chemosphere (2022) OpenAlex
R. Sardar, Shakil Ahmed, M. Akbar, Nasim Ahmad Yasin, Guihua Li - Chemosphere (2022) Semantic Scholar
A. Younis, Hebatollah Ismail - Egyptian Journal of Pure and Applied Science (2022) Semantic Scholar
Deleted Journal (2022) OpenAlex
Environmental Pollution (2022) Crossref
Plant Physiology and Biochemistry (2021) Crossref
Plant Physiology and Biochemistry (2021) OpenAlex
Aarifa Nabi, T. Aftab, M. Masroor, A. Khan, M. Naeem - Environmental Pollution (2021) Semantic Scholar
Environmental Pollution (2021) OpenAlex
Journal of Hazardous Materials (2021) Crossref
Journal of Hazardous Materials (2021) OpenAlex
Marek Vaculík, J. Kovac, I. Fialová, R. Fiala, Katarína Jašková et al. - Journal of Hazardous Materials (2021) Semantic Scholar
Hebatollah Ismail, A. Younis - Egyptian Academic Journal of Biological Sciences H Botany (2021) Semantic Scholar
Egyptian Academic Journal of Biological Sciences, H. Botany/Egyptian Academic Journal of Biological Sciences, H. Botany (2021) OpenAlex