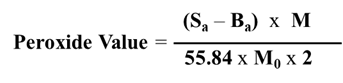Abstract
Insulin is a frequent peptide hormone addition in serum-free mammalian cell culture media. It contributes in a variety of biological functions, including as promoting cell proliferation, cell cycle progression, and glucose uptake. However, it is unknown how stable insulin is under in vitro cell culture media treatment conditions. The instability of insulin in aqueous solutions has caused a number of issues, necessitating the development of new therapeutic strategies that can keep insulin stable and functioning. Such choices are required to accommodate updated insulin delivery guidelines as well as the storage and transportation of insulin. To preserve structural and functional integrity, protein medicines are frequently stabilized with antioxidants in aqueous solutions. In the present study, the effects of the antioxidants disodium ethylenediaminetetraacetic acid dihydrate (EDTA) and sodium selenite (Se) and their ability to scavenge free radicals on insulin stability in the medium Dulbecco's Modified Eagle Medium (DMEM) and Roswell Park Memorial Institute (RPMI) were examined. To investigate the stability of human recombinant insulin, in vitro serum-free DMEM and RPMI media were utilized for 5 days at 37˚C containing different EDTA and Se concentrations. Reversed phase high performance liquid chromatography (RP-HPLC) was used to detect and quantify insulin. Sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) electrophoresis was used to assess conformational stability. The results demonstrated that, when EDTA and Se were added separately to DMEM and RPMI media, insulin stability was improved compared to when neither compound was added.
Author Contributions
Academic Editor: Loai Aljerf, Department of Life Sciences, Faculty of Dentistry, University of Damascus
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2023 A. S. Prakasha Gowda, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors confirm that this article content has no conflict of interest. This article does not contain any studies with human or animal subjects.
Citation:
Introduction
Insulin, a pancreatic hormone, is used to treat diabetes by regulating blood sugar levels. In addition, it serves as a growth factor for mammalian cell lines in vitro 1 and is added to culture media with hormone supplements for a variety of cell types to promote the optimal cell replication 2. The majority of proteins, including insulin, are not stable in aqueous solutions 3. A number of chemical modifications to an insulin's primary structure can drastically decrease its stability, resulting in insulin derivatives with modified secondary and quaternary structures that cause denaturation, polymerization, aggregation, and precipitation 4, 5, 6. This drastically reduces the biological functionality of the insulin, which causes considerable challenges for its use. In addition, a variety of environmental factors impact insulin's stability. For example, asparagine (A21) in the A chain of insulin deamidated in an acidic pH 7, as does asparagine (B3) in the B chain 8 and polymerization 9 in a neutral or alkaline environment. The in vitro accumulation of insulin has been associated with agitation 10, interaction with hydrophobic surfaces 11, variations in ionic strength 12, covalent aggregation by metal-catalyzed oxidation (MCO) 13, storage, and high temperatures 14, 15. In addition, high or low ambient temperatures cause insulin to form fibrils 16, 17.
The present study is interested in the use of insulin as a supplement in in vitro serum-free DMEM and RPMI media, as well as a practical method for detecting insulin stability in that medium. Insulin is naturally stored as a zinc-stabilized hexamer in healthy beta cells, and this has been replicated in most commercial insulin formulations to maintain insulin stability 18. However, excipients are frequently needed to maintain protein structure and function by enhancing the solubility, absorption, and stability in aqueous solutions but are frequently disregarded as the inactive ingredients in pharmaceutical formulations 19, 20. Alcohols, non-polar solvents, anionic and non-ionic surfactants, and different detergents were all utilized for investigating the physical stability of insulin in vitro21, but none of them have been found to be physiologically effective, safe, or practicable. But to keep insulin stable, the majority of insulin formulations included either phenol, meta-cresol, or both as excipients 22, 23. However, meta-cresol has been shown to cause immunological reactions 24, 25, and phenol and meta-cresol included in the formulation have been reported to cause cell death and inflammatory reactions 26. Additionally, citrate and EDTA used in the formulation of insulin prevent the reassembly of the monomer and dimer of insulin 27, while phenoxyethanol used in a variety of zinc-free formulations permits the stabilization of formulations with high insulin monomer contents 28. However, because most of these in vitro experiments are carried out in non-physiological conditions like acidic solutions or organic solvents, they often do not produce qualitatively favorable outcomes in cellular treatments. However, it is not yet known if insulin will remain stable when administered to in vitro cells. Therefore, it is important to understand in the present investigation how antioxidants could affect the stability of insulin in DMEM and RPMI media. In order to address this problem, EDTA and Se were chosen because they have antioxidant and radical-scavenging properties and may lessen the oxidative damage that occurs to insulin.
Several immunological and nonimmune techniques, including radioimmunoassay 29, enzyme immunoassay 30, luminous immunoassay and capillary electrophoresis 31, have been described for analyzing human insulin. However, the analysis of insulin and the byproducts of its breakdown in vivo and in vitro has increasingly utilized the use of RP-HPLC 32, 33, 34, 35. Furthermore, an HPLC method for the testing of insulin and A21 desamido insulin is included in the official monographs of the British and United States Pharmacopoeias 36, 37. In this investigation, DMEM and RPMI media without serum were used to independently examine the effects of EDTA and Se on insulin stability. The samples had been analyzed by RP-HPLC and SDS-PAGE as a secondary stability confirmation.
Experimental Materials
Insulin was purchased from MilliporeSigma (Burlington, MA United States). Disodium ethylenediaminetetraacetic acid dihydrate and Sodium selenite was purchased from VWR (Bridgeport, NJ, USA). Milli-Q water was used to prepare the EDTA and sodium Selenite stock solutions. Barium chloride dihydrate, Ferrous (F2+) sulfate heptahydrate, ferric (F3+) chloride hexahydrate and Ammonium thiocyanate are purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Methanol was received from Fisher Scientific (Fair Lawn, NJ, USA), Trifluoroacetic Acid, HPLC Grade was purchased from Sigma Aldrich (Burlington, MA, USA), 10N Hydrochloric Acid, was purchased from Ricca (Houston, TX , USA), 12 x 32 mm glass screw neck vial quick thread, LectraBond cap preslit PTFE/silicone septa was purchased from Waters (USA). 10X Tris-glycine SDS running buffer, simply blue safe stain, 2X sample buffer, SeeBlue Plus2 pre-Stained Standard and 16% gels were purchased from Life Technologies (Carlsbad, CA, USA). Dulbecco's Modified Eagle Medium and Roswell Park Memorial Institute 1640 medium were purchased from Life Technologies Corporation (Grand Island, NY, USA). Milli-Q water for solutions made in house with a Milli-Q system (Millipore, Milford, MA, USA). All other chemicals were obtained in an analytical grade or from standard commercial suppliers.
RP-HPLC procedure
The Agilent 1100 series, which includes a quaternary pump solvent delivery module, online degasser, thermostated column compartment, auto sampler, auto injector with 100 L injection loop, and a Variable Wavelength Detector, was used to perform the RP-HPLC. Prior to analysis, samples were kept in the autosampler at 5°C. The system was used in an HPLC lab at room temperature (20 ± 2°C). The 250 X 4 mm, 5 µm Nucleosil C18 column was used for the analysis, and the column temperature was maintained at 30 °C throughout. The mobile phase A contained 0.1% trifluoroacetic acid (TFA) in water and B 0.1% TFA in methanol. Gradient elution was used, and the program was set as follows: 40 - 90% B from 0 to 15 min. Injection volume was kept constant 25 μL. The flow rate of the mobile phase was set at 0.7 mL/min and the eluate was monitored at an UV wavelength of 214 nm. Chromatogram output, integration of peaks, calculation of peak areas and retention times were obtained using the Empower software, Version 3.
Impact of EDTA and Se on the stability of insulin in DMEM and RPMI medium
Since insulin only partially dissolves in pure water, 1 mg/mL of insulin was prepared in 0.01N hydrochloric acid (HCl) solution and stored in a refrigerator at 2 - 8 °C. To test the effect of EDTA and Se on insulin stability, insulin stock solution was diluted to 40 µg/mL in DMEM and RPMI media in the presence of different concentration of EDTA (0, 2.5, 5 and 10 mM) and Se (0, 2, 5 and 10 µM) in separate vials. Control samples were prepared at 40 µg/mL of insulin in DMEM and RPM media, and 0.01N HCl in separate vials without added EDTA and Se. All sample and control vials were incubated at 37 °C for 0 and 5 days. Following the individual RP-HPLC analysis of each sample solution, the linear regression of the analytical standard curve was used to calculate the % recovery of insulin from DMEM and RPMI media. The results of the insulin stability assays were compared with freshly prepared samples in DMEM, RPMI, 0.01N HCl, and control samples.
Effect of EDTA and Se on the level of peroxide in DMEM and RPMI medium
Preparation of a ferrous(II) chloride solution.
To prepare the ferrous(II) chloride solution, 0.5 g of ferrous(II) sulfate heptahydrate (FeSO4·7H2O) was dissolved in 49 mL of water. Approximately 0.4 g of barium chloride dihydrate (BaCl2·2H2O) was dissolved in 49 mL of water. Barium chloride solution was added slowly and with constant stirring to an ferrous(II) sulfate heptahydrate solution. Into this resulting solution, 2 mL of 10 N HCl was added, mixed well and allowed to precipitate of barium sulfate at room temperature for 20 to 30 minutes. The barium sulfate precipitate was filtered off through a 0.45 Micron filter to give a clear ferrous(II) chloride (FeCl2) solution, which was stored in a brown bottle at room temperature (RT).
Preparation of a standard ferric(III) chloride solution.
In order to make standard ferric(III) chloride (FeCl3), 0.4 g of ferric(III) chloride hexahydrate (FeCl3.6H2O) was dissolved in 49 mL of water, and 1 mL of 10 N HCl was then added. The solution was well mixed and stored at RT in a brown bottle.
Preparation of an ammonium thiocyanate solution.
The ammonium thiocyanate (NH4SCN) solution was made by dissolving 750 mg of ammonium thiocyanate in 2.5 mL of water, which was then stored at RT.
Procedure:To determine the peroxide value in DMEM and RPMI media, peroxide level was performed by modified IDF 38 method. For all the oxidation test reactions, stock insulin solution was diluted in DMEM and RPMI media to obtain a final insulin concentration of 40 µg/mL containing different concentration of EDTA (2.5, 5.0 and 10 mM) and Se (2.0, 5.0 and 10 µM). The control samples included of 40 µg/mL of insulin separately in DMEM and RPMI media without added EDTA or Se. The reaction was performed in 2 mL separate tubes covered with aluminum foil to protect the reaction mixture from light. After 5 days of incubation at 37 °C, 3 µL of ferrous(II) chloride (final concentration 15 µg/mL) was added, and followed by 15 µL of stock ammonium thiocyanate solution, which were then mixed well by vortex. The tubes were heated in a water bath at 50 °C for 2 minutes to promote color development. The tubes were placed in an ice bath for two minutes to reach RT 39. All tubes were wrapped in aluminum foil and left to sit at RT for 5 minutes, mixed well and transferred 200 µL from each sample tube into a 96-well plate. Using a spectrophotometer, the sample's (red color) absorbance was quickly measured at 505 nm against a blank that contained all of the reagents except insulin, EDTA, and Se. The results of the peroxide level were compared to control and freshly prepared insulin (40 g/mL) in DMEM and RPMI media that were free of EDTA or Se.
SDS-PAGE analysis of the stability of insulin
To determine the effect of EDTA and Se on insulin stability, 100 µg/mL insulin solution was made from insulin stock solution in DMEM and RPMI media with different concentrations of EDTA (0, 2.5, 5 and 10 mM) and Se (0, 2, 5 and 10 µM) in separate vials. All sample vials were incubated at 37 °C for 5 days. The stability of insulin was examined by SDS-PAGE under non-reduced conditions using tris-glycine sodium dodecyl sulfate (SDS) gel. As described earlier 40, before being put onto the gel for examination, samples of insulin stability were first mixed with sample buffer and heated the sample vials at 95 °C water bath for 10 minutes. From each sample tube 20 µL was loaded separately onto 16% gel. SeeBlue Plus2 pre-Stained Standard was used as the protein standard, and it was not further processed. Gels were run at RT in tris-glycine SDS buffer at a constant voltage (150V) until the dye front reached the end of the gel. SDS-PAGE gels were dyed using SimplyBlueTM Safe Stain, destained in 40 % methanol and washed twice with Milli-Q water. Using a densitometer, the gels were visualized.
Results
This study investigated at how the antioxidants and free radical scavengers EDTA and Se affected the stability of the insulin in the serum-free DMEM and RPMI media.
RP-HPLC analysis of insulin stability
The stability of insulin in DMEM and RPMI media was investigated under RP-HPLC analytical conditions. The literature has examined the physicochemical characteristics and chromatographic behavior of insulin. The effects of a specific combination of column type, mobile phase composition, and detection equipment were also investigated, in accordance with our prior analytical conditions 40. The maximum injection volume for future applications in biological sample analysis was set at 25 µL. The flow rate was adjusted to 0.7 mL/min to sharpen the peak during the study, which resulted in an insulin retention time of 13 minutes. This flow rate was found to be the best for reducing total run time while maintaining acceptable column backpressure. The column temperature was maintained at 30 °C to confirm that all of the components in the sample solution were successfully separated in 30 minutes. The detection wavelength of 214 nm was investigated for insulin while determining the detection wavelength for the analytical procedure, and it was found to yield peaks that were extremely sensitive and reproducible. In this optimized RP-HPLC condition, insulin separated successfully then was re-equilibrated to the starting condition. To better understand the impact of the DMEM and RPMI media matrix, chromatograms from 0.01 N HCl, DMEM, and RPMI were compared to those with insulin present. Each aqueous solution utilized in this study, both with and without insulin, was injected 25 µL at a time into the RP-HPLC apparatus. Insulin eluted as a single peak under the specified chromatographic conditions. As shown by peak purity analysis in(Figure 1), there seem to be no co-eluting peaks during the insulin retention time that could interfere with the peak of interest, implying that the insulin peak is pure.
Figure 1.DMEM and RPMI media matrix's impact on insulin analysis. Each aqueous solution was separately injected into the HPLC system with a volume of 25 µL. (A) 0.01 N HCl, (B) Insulin in 0.01 N HCl, (C) RPMI, (D) Insulin in RPMI, (E) DMEM and (F) insulin in DMEM. The chromatograms' output demonstrates that the analyte peak was pure, and there are no co-eluting peaks at the insulin peak's retention time to cause interference.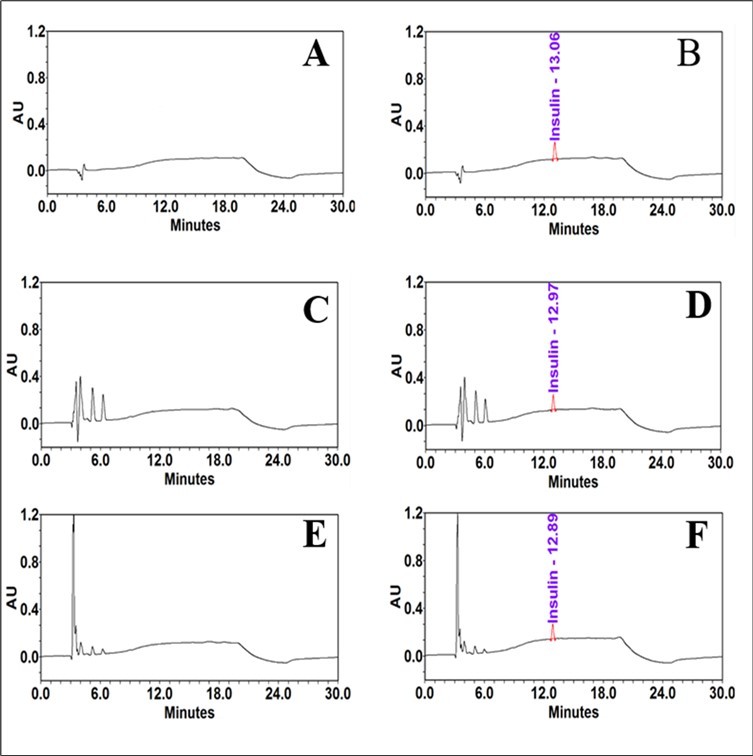
Analysis of linearity for the stability of RP-HPLC
Linearity is the ability of an analytical method to yield test results that are directly proportionate to the analyte concentration within a specific range. To evaluate the linearity of the insulin, a range of insulin concentrations (6, 12, 25, 40, 55, and 70 µg/mL) were used. The linearity was established using a linear regression analysis. Plotting the peak area obtained from the RP-HPLC against corresponding insulin concentrations resulted in the calibration curve. The standard curve for insulin was linear throughout the entire range and had a coefficient of determination (R2) of 0.9978 (Figure 2). This indicates a good linear relationship between all of the concentrations employed. The insulin y-intercepts were determined as an amount of the analytical concentration response. The peak area (Y) and the insulin concentration (X) are related by the standard curve's equation, y = 50000000x-35624, for this range.
Figure 2.Calibration curve for the quantification of standard insulin
The findings of the experiment demonstrated that the effects of different EDTA and se concentrations on insulin stability in DMEM and RPMI media were not concentration dependent, suggest that to maintain insulin stability in vitro with these media, 5 mM EDTA or 5 µM Se may be used. The results of this study showed that the antioxidants EDTA and Se had been used to make the insulin solution, proving that it was safe to use this approach to treat cells in DMEM and RPMI media. In addition, more research is required to fully understand both the short and long-term concentration dependent effects of EDTA and Se on insulin stability in DMEM and RPMI media.
Additionally, similar tests were carried out in DMEM and RPMI media containing higher EDTA and Se concentrations to see whether the insulin stability was EDTA and Se concentration-dependent. The results (data not supplied) show that adding more of these antioxidants had no apparent impact on insulin stability in either DMEM or RPMI media. Furthermore, EDTA and Se may change insulin bioactivity, suggesting that better insulin stability does not always translate into higher bioactivity. Since the bioactivity of insulin in the presence of EDTA or Se was not evaluated, the possibility that insulin may promote the survival and proliferation of an insulin-dependent cell line was not addressed in this study.
Investigating linearity to calculate peroxide value
A standard solution of ferric(III) chloride was made in water from stock ferric(III) chloride solution as described in the experimental section in order to produce the standard curve of Fe3+ concentration versus absorbance. Different levels of ferric iron concentrations (3, 5, 8, 12, 15 and 20 µg/mL) were examined to determine its linearity. The reaction was performed in 2 mL separate tubes covered with aluminum foil to protect the reaction mixture from light. Added 15 µL of stock ammonium thiocyanate solution, mixed well by vortex. The tubes were heated in a water bath at 50 °C for 2 minutes to promote color development. To bring the tubes temperature to RT, they were placed in an ice bath for 2 minutes 39. All tubes were wrapped in aluminum foil and left to sit at RT for 5 minutes. Mixed well and 200 µL of mixture is transferred from each tube into a 96-well plate. To establish the linearity, a linear regression analysis was utilized. The calibration graph was made by graphing the ferric iron concentrations against the absorbance obtained from the plate reader at 505 nm. The ferric iron coefficient of determination (R2) was determined to be 0.9959, the standard curve was linear over the entire range (Figure 3), and the results showed that the concentrations utilized closely followed Beer's law. The y-intercepts for ferric iron were calculated as a proportion of the analytical concentration response. The equation of the standard curve in this range, y = 0.0131x - 0.0239, relates the absorbance (Y) to the ferric iron concentration (X). The peroxide value, expressed as milliequivalents of peroxide per kilogram of sample was calculated by using the following equation.
Sa = absorbance of sample
Ba = absorbance of blank
M = slope of standard curve
M0 = mass in grams of the sample
55.84 = atomic weight of iron
The peroxide value is expressed as milliequivalents of peroxide rather than milliequivalents of oxygen using the division factor 2.
Figure 3.Calibration curve for the measuring of peroxide level
Peroxide levels are determined in DMEM and RPMI media
All lab solutions and media used for cell development have been found to be contaminated with transition metal ions 41, including DMEM, a cell culture medium that contains copper, iron(III) nitrate, free iron ions, and many other transition metal ions with strong pro-oxidant characteristics 42. Metal-catalyzed oxidation (MCO), however, is one of the therapeutically significant forms of oxidative stress-induced protein oxidation 43. Therefore, insulin is frequently susceptible to oxidative breakdown when made at relatively low concentrations of insulin in serum-free medium and other formulations, which can be an important concern. Although antioxidants are a family of compounds that can stop oxidation from occurring 42, they are generally lacking in cell culture media. Since the objective of this study was to determine if the antioxidant and radical-scavenger properties of EDTA and Se would decrease the levels of peroxide in the DMEM and RPMI media. As stated in the experimental section, insulin was incubated in DMEM and RPMI media with different concentrations of EDTA and Se for 5 days at 37 °C. The modified IDF method was used to determine the peroxide level, and results showed that EDTA considerably reduced the quantity of peroxide level in both DMEM and RPMI media compared to the control (Table 3, Table 4 and Figure 4, Figure 5). This demonstrated that EDTA might reduce solution-induced protein oxidation by peroxide and Fe(II) 44, and it's probable that it will interact with free radicals and metal ions in DMEM and RPMI media to prevent the chain reaction before it impacts insulin. It can also be thought of as a chelating agent to remove redox metal ions from DMEM and RPMI media to prevent the oxidation of insulin. The results of the experiment, which showed that there was no dose-dependent effect of different EDTA concentrations on the decreased peroxide levels in DMEM and RPMI media, therefore 5 mM EDTA may be used in DMEM and RPMI media in addition to insulin formulations to prevent insulin oxidation. Additional research is also required in order to completely explain the hypotheses underlying the EDTA-induced short- and long-term concentration-dependent effects on reduced peroxide levels in DMEM and RPMI media. Although Se being a necessary trace element as well as essential for antioxidant defense 45, this investigation found that Se had no effect on peroxide levels in the DMEM or RPMI media when compared to control. However, Se may initially produce superoxide in vitro after being added to the medium in both DMEM and RPMI 46. Therefore, it may suggest that Se only functions as an antioxidant in vivo when it gets incorporated into selenoproteins and not when it is present in in vitro DMEM or RPMI media alone.
Figure 4.Peroxide levels in DMEM media are affected by EDTA and Se. Separate vials containing different concentrations of EDTA and Se were used for preparing the insulin in DMEM medium. The stability sample vials were incubated at 37 °C for five days. Using equation, the peroxide level was determined as milliequivalents of peroxide per kilogram of sample. Results from three different trials were compared to controls and newly made insulin in DMEM medium. The results represent the mean of three different experiments.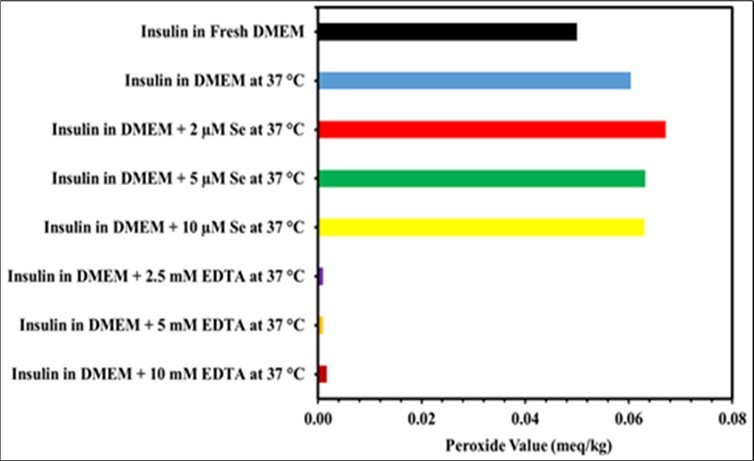
Figure 5.Peroxide levels in RPMI media are affected by EDTA and Se. Separate vials containing different concentrations of EDTA and Se were used for preparing the insulin in RPMI medium. The stability sample vials were incubated at 37 °C for five days. Using equation, the peroxide level was determined as milliequivalents of peroxide per kilogram of sample. Results from three different trials were compared to controls and newly made insulin in RPMI medium. The results represent the mean of three different experiments.
SDS-PAGE study confirms the stability of insulin
In order to confirm the RP-HPLC findings, we performed an additional stability-indicating SDS-PAGE experiment to examine the impact of EDTA and Se on insulin stability in DMEM and RPMI media. In DMEM and RPMI media with different concentrations of EDTA and Se, SDS-PAGE was performed to determine the stability of insulin for 5 days, as described in the experimental section. Insulin migrated in a single band with a molecular weight of approximately 5 kDa in all stability tests under non-redu cing conditions. Insulin stability studies in DMEM and RPMI media containing antioxidants at different concentrations are shown in Figure 7. The results demonstrated that, in comparison to the control, insulin stability did not decrease in either the DMEM or RPMI media. The insulin bands' intensity did not change considerably over time. Additionally, not all insulin stability samples under non-reduced conditions showed visible precipitates and dimer bands, indicating the absence of insulin fibrils, disulfide and non-disulfide cross-linking interaction. Soluble or insoluble aggregates, reversible or irreversible aggregates, covalent or noncovalent aggregates are all possibilities 47. In the absence of protein aggregation bands, precipitates most likely formed physically and may have completely disintegrated in the SDS sample buffer under sample conditions. However, the SDS-PAGE result is in agreement with the HPLC single peak, suggesting that the presence of EDTA and Se in DMEM and RPMI media protected the insulin from oxidation.
Discussion
It is widely known that insulin stability has a direct impact on human health, and both pharmaceutical companies and academic institutions routinely measure insulin levels. Since mammalian cells are routinely cultivated in vitro using readily available, chemically defined media, which are frequently extremely complicated and have been designed to encourage cell multiplication. It is unclear how long insulin will remain stable at 37 °C when used as a growth factor in cell culture medium. Moreover, no research has specifically focused on the stability of insulin in DMEM and RPMI media, the objective of this study was to evaluate how well insulin could maintain its stability in both DMEM and RPMI media in the presence of antioxidants EDTA and Se. Then, using a modified IDF method, these compounds were tested for their ability to lower peroxide levels in DMEM and RPMI media. The insulin bioassay was insensitive, therefore the soluble insulin concentration in DMEM and RPMI media was measured using the RP-HPLC method.
Since the insulin samples were made from DMEM and RPMI media, the samples' matrix required to be free of any impediments to insulin detection for the length of the 30-minute run time. Using our chromatographic approach, insulin was eluted from DMEM and RPMI media in a single peak at 13 minutes (Figure 1). The concentration of soluble insulin in the stability samples with EDTA and Se in DMEM and RPMI media increased in comparison to the control (Table 1 and Table 2), even though RP-HPLC was unable to find any degradation products, such as monomer, covalent dimerization, or high-molecular-weight oligomers, throughout the stability analysis. It is anticipated that limiting insulin solubility, ionic complexation, salting out, and charge neutrality around the isoelectric pH will result in some additional non-covalent aggregation formation. Since non-covalent aggregates will dissociate in the organic solvent-based mobile phase's low pH, it is difficult to identify them. However, in this result neither the DMEM nor the RPMI media showed any evidence of insulin that had been degraded or oxidized in the presence of EDTA and Se. Overall, these results demonstrate that, when used as in vitro treatments, 5 mM EDTA or 5 µM Se can stabilize insulin in DMEM and RPMI conditions for up to 5 days. Moreover, when the highest concentrations of EDTA and Se were tested, there were no noticeable differences in the insulin stability between the DMEM and RPMI media (data not shown).
Table 1. Impact of EDTA and Se on the stability of insulin in DMEM media. In separate vials, insulin solutions were prepared in DMEM media, in the presence of different concentration of Se and EDTA. Sample vials were incubated at 37 °C for 5 days. The solutions were analyzed separately by RP-HPLC and the percent recovery of insulin was determined by using the Empower software, version 3. The results represent the mean of three different experiments.| Insulin Samples | % Insulin recovered | |
|---|---|---|
| 0 Day | 5 Days at 37 °C | |
| Insulin in 0.01 N Hcl | 102.8 | 96.8 |
| Insulin in DMEM | 95 | 75.3 |
| Insulin in DMEM + 2 µM Se | 93.9 | 84.1 |
| Insulin in DMEM + 5 µM Se | 95.7 | 87.4 |
| Insulin in DMEM + 10 µM Se | 95.2 | 86.0 |
| Insulin in DMEM + 2.5 mM EDTA | 93.6 | 83.5 |
| Insulin in DMEM + 5 mM EDTA | 93.6 | 93.3 |
| Insulin in DMEM + 10 mM EDTA | 94.9 | 95.1 |
| Insulin Samples | % Insulin recovered | |
|---|---|---|
| 0 Day | 5 Days at 37 °C | |
| Insulin in RPMI | 94 | 79.8 |
| Insulin in RPMI + 2 µM Se | 96.6 | 88.8 |
| Insulin in RPMI + 5 µM Se | 99.6 | 100.8 |
| Insulin in RPMI + 10 µM Se | 97.6 | 91.5 |
| Insulin in RPMI + 2.5 mM EDTA | 98.6 | 93.5 |
| Insulin in RPMI + 5 mM EDTA | 97.9 | 99.7 |
| Insulin in RPMI + 10 mM EDTA | 99.1 | 92.1 |
Additionally, the level of peroxide was measured in the presence of antioxidants EDTA and Se in both DMEM and RPMI media. Due to the lack of antioxidants in cell culture media, free radical production is constant in in vitro cell culture, where the cells are maintained at oxygen (O2) concentrations higher than in vivo48, exposed to different physiochemical conditions 49, photochemically reducing dissolved organic matter 50, and having metal ions in DMEM and RPMI media 51. Therefore in the aqueous solutions the protein oxidation can be induced by radical species 52. In view of the current research status, the objective of this research was to determine the peroxide levels in DMEM and RPMI media independently and in the presence of different EDTA and Se concentrations. To accomplish this, the peroxide assay was carried out using a modified IDF method. The results shown in (Table 3, Table 4 and Figure 4, Figure 5) demonstrate that antioxidant EDTA treatment of insulin in both DMEM and RPMI media considerably decreased the level of peroxide. The result was compared with a freshly prepared and control samples. According to the results of this experimental study, 5 mM of EDTA can lower peroxide levels by scavenging free radicals and chelated metal ions that are present in DMEM and RPMI media.
Table 3. Peroxide levels in DMEM media are affected by EDTA and Se. The effect of the antioxidants EDTA and Se on the peroxide level in serum-free DMEM medium was studied. After preparing the insulin solution in DMEM media containing different EDTA and se concentrations, it was incubated for five days at 37 °C. The amount of peroxide was calculated as milliequivalents of peroxide per kilogram of sample using equation. When compared to the control and untreated sample, the antioxidant EDTA dramatically reduced the level of peroxide in DMEM media. Se, however, had no impact on the amount of peroxide present in DMEM media. The results represent the mean of three different experiments.| Sample name | Peroxide Value (meq/kg) | |
|---|---|---|
| 0 day | 5 days at 37 °C | |
| Insulin prepared in fresh DMEM | 0.05 | - |
| Insulin in DMEM | - | 0.06 |
| Insulin in DMEM + 2 µM Se | - | 0.067 |
| Insulin in DMEM + 5 µM Se | - | 0.063 |
| Insulin in DMEM + 10 µM Se | - | 0.063 |
| Insulin in DMEM + 2.5 mM EDTA | - | 0.001 |
| Insulin in DMEM + 5 mM EDTA | - | 0.001 |
| Insulin in DMEM + 10 mM EDTA | - | 0.002 |
| Sample name | Peroxide Value (meq/kg) | |
|---|---|---|
| 0 day | 5 days at 37 °C | |
| Insulin prepared in fresh RPMI | 0.052 | - |
| Insulin in RPMI | - | 0.071 |
| Insulin in RPMI + 2 µM Se | - | 0.063 |
| Insulin in RPMI + 5 µM Se | - | 0.072 |
| Insulin in RPMI + 10 µM Se | - | 0.077 |
| Insulin in RPMI + 2.5 mM EDTA | - | 0.007 |
| Insulin in RPMI + 5 mM EDTA | - | 0.004 |
| Insulin in RPMI + 10 mM EDTA | - | 0.002 |
The peroxide levels in the Se treated insulin samples increased similarly to the control samples and did not decrease in either the DMEM or RPMI media. The investigation's findings supported the hypothesis that selenite would generate superoxide in vitro before producing other ROS 46. Selenite can oxidize by photochemically producing hydroxyl radicals (●OH) and hydrogen peroxide (H2O2) 53, as well as by varying the concentration of Se, the pH, the ionic strength, and the medium 54. Although selenium has antioxidant properties 55, experimental findings in DMEM and RPMI medium show that Se had no antioxidant impact in vitro. The present investigation also suggests that Se is a non-metallic element with chemical bonding properties and an electronic configuration that is similar to sulfur. Se is more nucleophilic than sulfur, hence they are frequently employed interchangeably in chemical processes. Due to this characteristic, selenium is more electrophile reactive. However, Se might react with the cysteine in the A chain of insulin and produce Se-insulin, which could increase insulin stability and reduce oxidative damage 56. To fully understand the Se peroxide-reducing ability in in vitro DMEM and RPMI media, however, more research is required. The results of the present investigation suggest that EDTA can reduce peroxide levels in cell culture media like DMEM and RPMI. Due to its proven ability to improve insulin stability and reduce peroxide levels in DMEM and RPMI media, 5 mM EDTA may be a potential therapeutic approach for the treatment of insulin in in vitro cell culture.
Figure 6.Effect of EDTA and Se on the stability of insulin in DMEM medium.Different concentrations of Se and EDTA were used for preparing insulin in DMEM medium in separate vials, and each sample vial was incubated at 37 °C for 5 days. Tris-glycine SDS 16% gel was used in SDS-PAGE to analyze the stability of the insulin under non-reduced conditions, and SimplyBlueTM Safe Stain was used for staining. Three different experiments were carried out.
The conformational stability of insulin samples in DMEM and RPMI media containing different concentrations of EDTA and Se has been evaluated using SDS-PAGE under non-reduced conditions. The intensity of the insulin bands remained unchanged in any of the stability samples. The lack of deaminated, dimers, or degraded products in all samples implies that throughout the stability test in DMEM and RPMI media, insulin did not undergo an oxidation reaction or disulfide cross-linking.
Figure 7.Effect of EDTA and Se on the stability of insulin in RPMI medium. Different concentrations of Se and EDTA were used for preparing insulin in RPMI medium in separate vials, and each sample vial was incubated at 37 °C for 5 days. Tris-glycine SDS 16% gel was used in SDS-PAGE to analyze the stability of the insulin under non-reduced conditions, and SimplyBlueTM Safe Stain was used for staining. Three different experiments were carried out.
Conclusions
The purpose of this study was to evaluate the stability of insulin in the presence of the antioxidants EDTA and Se in DMEM and RPMI media. It was found that insulin stability improved when EDTA and Se were added individually to the DMEM and RPMI media. Since the impact of different EDTA and Se concentrations on the stability of insulin in DMEM and RPMI medium was not concentration dependent, it is suggested that 5 mM EDTA or 5 µM Se may be used to maintain insulin stability in vitro using these media. In addition, the level of peroxide was measured separately in the presence of EDTA and Se in both DMEM and RPMI media. The addition of EDTA substantially decreased the amount of peroxide in both DMEM and RPMI media, but Se had no antioxidant effects on either DMEM or RPMI media. The experiment's findings, which demonstrated that there was no concentration-dependent relationship between the effects of different EDTA concentrations on peroxide levels in DMEM and RPMI media. Thus, 5 mM EDTA can be added to DMEM and RPMI media in addition to insulin formulations in order to reduce the peroxide level and prevent insulin oxidation. In addition, the results of the study suggested that although Se does not have an antioxidant effect, it would be possible to add 5 µM Se to DMEM and RPMI media in order to maintain insulin stability during in vitro treatments.
Further research is required to fully understand the mechanisms behind the short- and long-term concentration-dependent effects of EDTA on reduced peroxide levels in DMEM and RPMI media.
Authors Contributions
A.S. Prakash Gowda conceived, planned, carried out the experiments, and wrote the manuscript. The manuscript was reviewed by Andrew D. Schaefer and Terry K. Schuck. All authors were given approval for publication.
Acknowledgments
The authors wish to thank the management of Department of raw materials, Eurofins BioPharma Product Testing, Lancaster, for supporting this work.
Funding Support
This research received no external funding. This project was supported by Department of raw materials, Eurofins BioPharma Product Testing, Lancaster, PA 17601 USA
Abbreviations:
Sodium Selenite (Se)
References
- 2.Sato Gordon, Arthur Beck Pardee, David Andrew Sirbasku. (1982) Growth of Cells in Hormonally Defined Media. Google Books ·. 2 v. (xxx, 1214 p.): ill. ; 27 cm. · 0879691565.
- 3.Sluzky V, Tamada J A, Klibanov A M, Langer R. (1991) Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci U S A 88(21), 9377-81.
- 4.Phillips N B, Whittaker J, Ismail-Beigi F, Weiss M A. (2012) Insulin fibrillation and protein design: topological resistance of single-chain analogs to thermal degradation with application to a pump reservoir. J Diabetes Sci Technol. 6(2), 277-88.
- 5.M C, D K Chou, B M Murphy, R W Payne, D S Katayama.Stability of protein pharmaceuticals: An update. , Pharm. Res 2010, 544-575.
- 7.Haas J, Vöhringer-Martinez E, Bögehold A, Matthes D, Hensen U et al. (2009) Primary steps of pH-dependent insulin aggregation kinetics are governed by conformational flexibility. Chembiochem. 10(11), 1816-22.
- 8.Brange J, Langkjaer L, Havelund S, Vølund A. (1992) Chemical stability of insulin. 1. Hydrolytic degradation during storage of pharmaceutical preparations. Pharm Res. 9(6), 715-26.
- 9.Brange J, Havelund S, Hougaard P. (1992) Chemical stability of insulin. 2. Formation of higher molecular weight transformation products during storage of pharmaceutical preparations. Pharm Res. 9(6), 727-34.
- 10.Malik R, Roy I. (2011) Probing the mechanism of insulin aggregation during agitation. , Int 73-80.
- 11.Nault L, Guo P, Jain B, Bréchet Y, Bruckert F et al. (2013) Human insulin adsorption kinetics, conformational changes and amyloidal aggregate formation on hydrophobic surfaces. Acta Biomater. Feb;9(2): 5070-9. doi: 10.1016/j.actbio.2012.09.025. Epub 23022543.
- 12.Buell A K, Hung P, Salvatella X, Welland M E, Dobson C M et al. (2013) Electrostatic effects in filamentous protein aggregation. , Biophys 104(5), 1116-26.
- 13.Torosantucci R, Mozziconacci O, Sharov V, Schöneich C, Jiskoot W. (2012) Chemical modifications in aggregates of recombinant human insulin induced by metal-catalyzed oxidation: covalent cross-linking via michael addition to tyrosine oxidation products. Pharm Res. 29(8), 2276-93.
- 14.Waldemar O Storvick, Harry J Henry. (1968) . , Effect of Storage Temperature on Stability of Commercial Insulin Preparations. Diabetes 1, 499-502.
- 15.Vimalavathini R, Gitanjali B. (2009) Effect of temperature on the potency & pharmacological action of insulin. Indian J Med Res. 130(2), 166-9.
- 16.Ahmad A, Millett I S, Doniach S, Uversky V N, Fink A L.Partially folded intermediates in insulin fibrillation. , Biochemistry 42(39), 11404-16.
- 17.Kurouski D, Washington J, Ozbil M, Prabhakar R, Shekhtman A et al.Disulfide bridges remain intact while native insulin converts into amyloid fibrils. , PLoS One 7(6).
- 18.Xu Y, Yan Y, Seeman D, Sun L, Dubin P L. (2012) Multimerization and aggregation of native-state insulin: effect of zinc. 10.1021/la202902a. Epub 2011 Dec 5. PMID: , Langmuir 28(1), 579-86.
- 19.Kamerzell T J, Esfandiary R, Joshi S B, Middaugh C R, Volkin D B. (2011) Protein-excipient interactions: mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. Oct;63(13): 1118-59. doi: 10.1016/j.addr.2011.07.006. Epub 21855584.
- 20.Rayaprolu B M, Strawser J J, Anyarambhatla G. (2018) Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev Ind Pharm. Epub 44(10), 1565-1571.
- 21.Lougheed W D, Albisser A M, Martindale H M, Chow J C, Clement J R. (1983) Physical stability of insulin formulations. Diabetes. 32(5), 424-32.
- 22.Gualandi-Signorini A M, Giorgi G. (2001) Insulin formulations--a review. Eur Rev Med Pharmacol Sci. 5(3), 73-83.
- 23.Teska B M, Alarcón J, Pettis R J, Randolph T W, Carpenter J F. (2014) Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J Pharm Sci. Aug;103(8): 2255-67. doi: 10.1002/jps.24039. Epub 6, 24909933.
- 24.Wheeler B J, Taylor B J. (2012) Successful management of allergy to the insulin excipient metacresol in a child with type 1 diabetes: a case report. J Med Case Rep. 31, 10-1186.
- 25.Clerx V, Keybus C Van Den, Kochuyt A, Goossens A. (2003) Drug intolerance reaction to insulin therapy caused by metacresol. Contact Dermatitis. 48(3), 162-3.
- 26.Weber C, Kammerer D, Streit B, Licht A H. (2014) Phenolic excipients of insulin formulations induce cell death, pro-inflammatory signaling and MCP-1 release. Toxicol Rep. 6, 194-202.
- 27.Pohl R, Hauser R, Li M, E De Souza, Feldstein R et al. (2012) Ultra-rapid absorption of recombinant human insulin induced by zinc chelation and surface charge masking. J Diabetes Sci Technol. 6(4), 755-63.
- 28.Caitlin L Maikawa, Leslee T Nguyen, Joseph L Mann, Eric A Appel.Formulation excipients and their role in insulin stability and association state in formulation. bioRxiv. 2022.08.01.502380; doi: https://doi.org/10.1101/2022.08.01.502380
- 30.Zaitsu K, Kimura Y, Ohba Y. (1999) Heme-Undecapeptide Labeling on Insulin for the Immunoassay of Insulin with Chemiluminescence Detection. https://doi.org/10.2116/analsci.15.871 , ANAL. SCI 15, 871-878.
- 31.German I, Kennedy R T. (2000) Rapid simultaneous determination of glucagon and insulin by capillary electrophoresis immunoassays. , J Chromatogr B Biomed Sci Appl 742(2), 353-62.
- 32.Jacob Dolly, M Joan Taylor, Tomlins Paul, Sahota Tarsem. (2019) Insulin Solution Stability and Biocompatibility with Materials Used for an Implantable Insulin Delivery Device Using Reverse Phase HPLC Methods". , Applied Sciences 9, 4794-10.
- 33.D S Rajan, K V Gowda, Mandal U, Ganesan M, Bose A et al. (2006) Development of RP-HPLC for analysis of human insulin. , Ind. J. Pharm. Sci 68, 662-665.
- 34.Yilmaz Bilal. (2012) Yucel Kadioglu, Ilyas Capoglu, Determination of Insulin in Humans with Insulin-Dependent Diabetes Mellitus Patients by HPLC with Diode Array Detection. , Journal of Chromatographic Science 50, 586-590.
- 35.Oliva A, Fariña J, Llabrés M. (2000) Development of two high-performance liquid chromatographic methods for the analysis and characterization of insulin and its degradation products in pharmaceutical preparations. , J Chromatogr B Biomed Sci Appl 749(1), 25-34.
- 36.Pharmacopoeia British. (2014) Formulated Preparations: Specific Monographs: Insulin Preparations. Available online: https://www.pharmacopoeia.com/ 3.
- 37. (2006) The United States Pharmacopoeia/National Formulary 2006, 29th ed.; USP29-NF24; Twinbrook Parkway:. , Rockville, MD, USA
- 38.Nalur C Shantha. (1994) Eric A Decker, Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. , Journal of AOAC INTERNATIONAL 77, 421-424.
- 39.C M Stine, H A, S T Coulter, Jenness R. (1954) A Modified Peroxide Test for Detection of Lipid Oxidation in Dairy Products. , Journal of Dairy Science 37, 202-208.
- 40.Gowda A S Prakasha, Andrew D Schaefer, Terry K Schuck. (2021) . Effect of Excipients on Recombinant Interleukin-2 Stability in Aqueous Buffers.American Journal of Analytical Chemistry. Vol.12 No.10 DOI: 10.4236/ajac.2021.1210022 .
- 42.Halliwell B. (2003) Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 3-6.
- 43.Stadtman E R, Oliver C N. (1991) Metal-catalyzed oxidation of proteins. Physiological consequences. , J Biol Chem 266(4), 2005-8.
- 44.Methionine tryptophan. (2009) and histidine oxidation in a model protein, PTH: mechanisms and stabilization. J Pharm Sci. 98(12), 4485-500.
- 45.Zimmerman M T, Bayse C A, Ramoutar R R, Brumaghim J L. (2015) Sulfur and selenium antioxidants: challenging radical scavenging mechanisms and developing structure-activity relationships based on metal binding. J Inorg Biochem. Apr;145: 30-40. doi: 10.1016/j.jinorgbio.2014.12.020. Epub 25600984.
- 46.Misra S, Boylan M, Selvam A, Spallholz J E, Björnstedt M. (2015) Redox-active selenium compounds--from toxicity and cell death to cancer treatment. Nutrients. 7(5), 3536-56.
- 47.M E Cromwell, Hilario E, Jacobson F. (2006) Protein Aggregation and Bioprocessing. , The AAPS Journal 8, 572-579.
- 48.Chwa M, Atilano S R, Reddy V, Jordan N, Kim D W et al.Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts.Invest Ophthalmol Vis Sci. 2006, 1902-10.
- 49.Lobo V, Patil A, Phatak A, Chandra N. (2010) Free radicals, antioxidants and functional foods: Impact on human health,”. , Pharmacognosy Reviews 4, 118-126.
- 50.Garg Shikha, Andrew L Rose, T David Waite. (2011) Photochemical production of superoxide and hydrogen peroxide from natural organic matter. , Geochimica et Cosmochimica Acta, Volume 75, Issue 15, 4310-4320.
- 51.Stohs S J, Bachi D. (1995) Oxidative mechanisms in the toxicity of metal ions. , Free Radical biology and Medicine 2, 321-336.
- 52.Dean R T, Fu S, Stocker R, Davies M J.Biochemistry and pathology of radical-mediated protein oxidation. , Biochem J 1997, 1-18.
- 53.Paydary Pooya, Schellenger Alexandra E P, Teli Minerva, Deb P Jaisi. (2021) Annalisa Onnis-Hayden, Philip Larese-Casanova, Chemical oxidation of selenite to selenate: Evaluation of reactive oxygen species and O transfer pathways, Chemical Geology, Volume 575. 120229-0009.
- 54.Gruebel K A, Davis J A, Leckie J O. (1995) Kinetics of oxidation of selenite to selenate in the presence of oxygen, titania, and light.Environ Sci Technol. 29(3), 586-94.